Amines are prepared by the following methods:
1. Reduction of nitro compounds
Nitro compounds are reduced to amines by passing hydrogen gas in the presence of finely divided nickel, palladium or platinum and also by reduction with metals in acidic medium. Nitroalkanes can also be similarly reduced to the corresponding alkanamines.
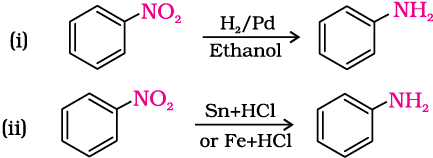
Reduction with iron scrap and hydrochloric acid is preferred because FeCl2 formed gets hydrolysed to release hydrochloric acid during the reaction. Thus, only a small amount of hydrochloric acid is required to initiate the reaction.
2. Ammonolysis of alkyl halides
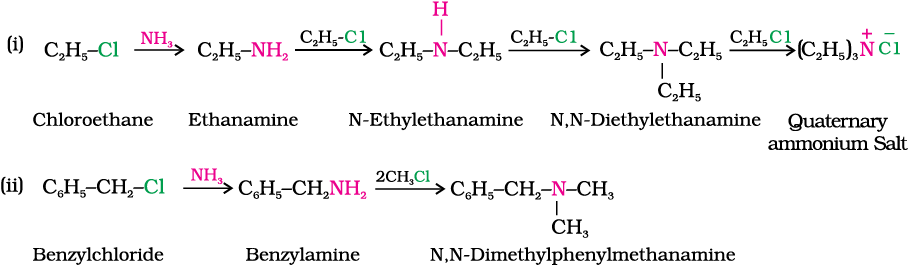
You have read (Unit 10, Class XII) that the carbon - halogen bond in alkyl or benzyl halides can be easily cleaved by a nucleophile. Hence, an alkyl or benzyl halide on reaction with an ethanolic solution of ammonia undergoes nucleophilic substitution reaction in which the halogen atom is replaced by an amino (–NH2) group. This process of cleavage of the C–X bond by ammonia molecule is known as ammonolysis. The reaction is carried out in a sealed tube at 373 K. The primary amine thus obtained behaves as a nucleophile and can further react with alkyl halide to form secondary and tertiary amines, and finally quaternary ammonium salt.
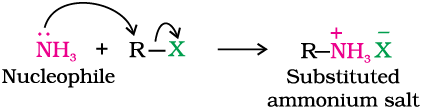
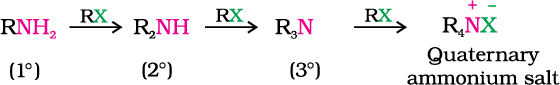
The free amine can be obtained from the ammonium salt by treatment with a strong base:
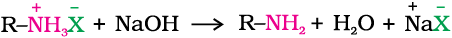
Ammonolysis has the disadvantage of yielding a mixture of primary, secondary and tertiary amines and also a quaternary ammonium salt. However, primary amine is obtained as a major product by taking large excess of ammonia.
The order of reactivity of halides with amines is RI > RBr >RCl.
Example 13.1
Write chemical equations for the following reactions:
(i) Reaction of ethanolic NH3 with C2H5Cl.
(ii) Ammonolysis of benzyl chloride and reaction of amine so formed with two moles of CH3Cl.
Solution
3. Reduction of nitriles
Nitriles on reduction with lithium aluminium hydride (LiAlH4) or catalytic hydrogenation produce primary amines. This reaction is used for ascent of amine series, i.e., for preparation of amines containing one carbon atom more than the starting amine.
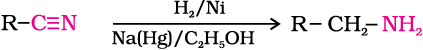
4. Reduction of amides
The amides on reduction with lithium aluminium hydride yield amines.
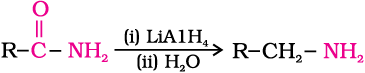
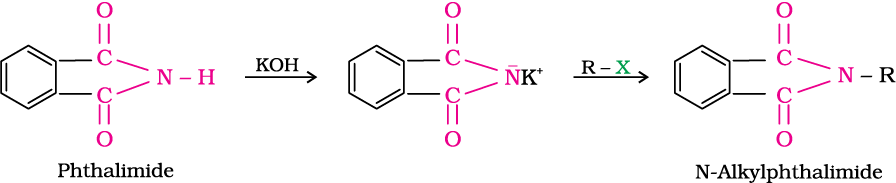
5. Gabriel phthalimide synthesis
Gabriel synthesis is used for the preparation of primary amines. Phthalimide on treatment with ethanolic potassium hydroxide forms potassium salt of phthalimide which on heating with alkyl halide followed by alkaline hydrolysis produces the corresponding primary amine. Aromatic primary amines cannot be prepared by this method because aryl halides do not undergo nucleophilic substitution with the anion formed by phthalimide.
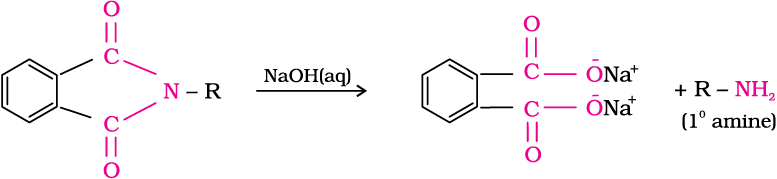
6. Hoffmann bromamide degradation reaction
Hoffmann developed a method for preparation of primary amines by treating an amide with bromine in an aqueous or ethanolic solution of sodium hydroxide. In this degradation reaction, migration of an alkyl or aryl group takes place from carbonyl carbon of the amide to the nitrogen atom. The amine so formed contains one carbon less than that present in the amide.
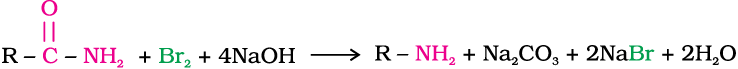
Example 13.2
Write chemical equations for the following conversions:
(i) CH3–CH2–Cl into CH3–CH2–CH2–NH2
(ii) C6H5–CH2–Cl into C6H5–CH2–CH2–NH2
Solution
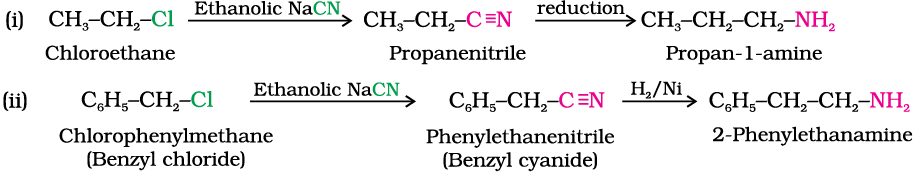
Example 13.3
Write structures and IUPAC names of
(i) the amide which gives propanamine by Hoffmann bromamide reaction.
(ii) the amine produced by the Hoffmann degradation of benzamide.
Solution
(i) Propanamine contains three carbons. Hence, the amide molecule must contain four carbon atoms. Structure and IUPAC name of the starting amide with four carbon atoms are given below:

Butanamide
(ii) Benzamide is an aromatic amide containing seven carbon atoms. Hence, the amine formed from benzamide is aromatic primary amine containing six carbon atoms.
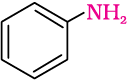 Aniline or benzenamine
Aniline or benzenamine
Intext Question
13.3 How will you convert
(i) Benzene into aniline (ii) Benzene into N, N-dimethylaniline
(iii) Cl–(CH2)4–Cl into hexan-1,6-diamine?