Glucose is an aldohexose and is also known as dextrose. It is the monomer of many of the larger carbohydrates, namely starch, cellulose. It is probably the most abundant organic compound on earth. It was assigned the structure given below on the basis of the following evidences:
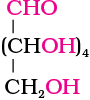
Glucose
1. Its molecular formula was found to be C6H12O6.
2. On prolonged heating with HI, it forms n-hexane, suggesting that all the six carbon atoms are linked in a straight chain.
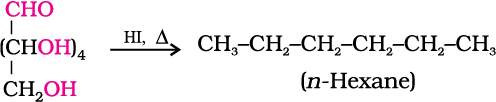
3. Glucose reacts with hydroxylamine to form an oxime and adds a molecule of hydrogen cyanide to give cyanohydrin. These reactions confirm the presence of a carbonyl group (>C = O) in glucose.
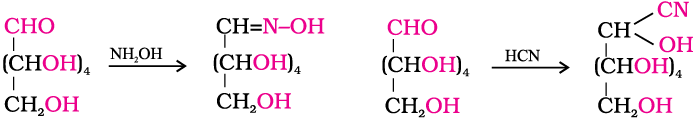
4. Glucose gets oxidised to six carbon carboxylic acid (gluconic acid) on reaction with a mild oxidising agent like bromine water. This indicates that the carbonyl group is present as an aldehydic group.
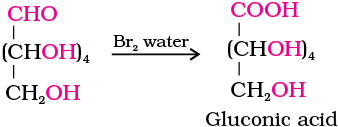
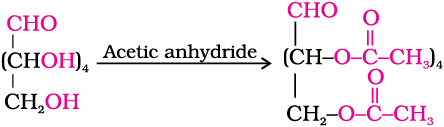
5. Acetylation of glucose with acetic anhydride gives glucose pentaacetate which confirms the presence of five –OH groups. Since it exists as a stable compound, five –OH groups should be attached to different carbon atoms.
6. On oxidation with nitric acid, glucose as well as gluconic acid both yield a dicarboxylic acid, saccharic acid. This indicates the presence of a primary alcoholic (–OH) group in glucose.
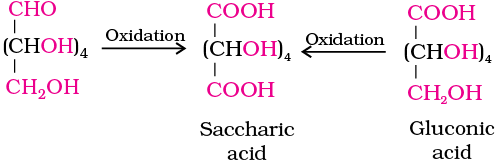
The exact spatial arrangement of different —OH groups was given by Fischer after studying many other properties. Its configuration is correctly represented as I. So gluconic acid is represented as II and saccharic acid as III.
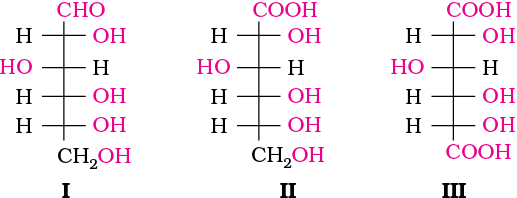
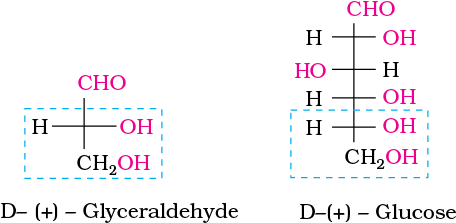
Glucose is correctly named as D(+)-glucose. ‘D’ before the name of glucose represents the configuration whereas ‘(+)’ represents dextrorotatory nature of the molecule. It should be remembered that ‘D’ and ‘L’ have no relation with the optical activity of the compound. They are also not related to letter ‘d’ and ‘l’ (see Unit 10). The meaning of D– and L– notations is as follows.
The letters ‘D’ or ‘L’ before the name of any compound indicate the relative configuration of a particular stereoisomer of a compound with respect to configuration of some other compound, configuration of which is known. In the case of carbohydrates, this refers to their relation with a particular isomer of glyceraldehyde. Glyceraldehyde contains one asymmetric carbon atom and exists in two enantiomeric forms as shown below.
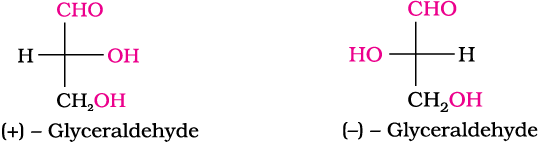
(+) Isomer of glyceraldehyde has ‘D’ configuration. It means that when its structural formula is written on paper following specific conventions which you will study in higher classes, the –OH group lies on right hand side in the structure. All those compounds which can be chemically correlated to D (+) isomer of glyceraldehyde are said to have D-configuration whereas those which can be correlated to ‘L’ (–) isomer of glyceraldehyde are said to have L—configuration. In L (–) isomer –OH group is on left hand side as you can see in the structure. For assigning the configuration of monosaccharides, it is the lowest asymmetric carbon atom (as shown below) which is compared. As in (+) glucose, —OH on the lowest asymmetric carbon is on the right side which is comparable to (+) glyceraldehyde, so (+) glucose is assigned D-configuration. Other asymmetric carbon atoms of glucose are not considered for this comparison. Also, the structure of glucose and glyceraldehyde is written in a way that most oxidised carbon (in this case –CHO)is at the top.
The structure (I) of glucose explained most of its properties but the following reactions and facts could not be explained by this structure.
1. Despite having the aldehyde group, glucose does not give Schiff’s test and it does not form the hydrogensulphite addition product with NaHSO3.
2. The pentaacetate of glucose does not react with hydroxylamine indicating the absence of free —CHO group.
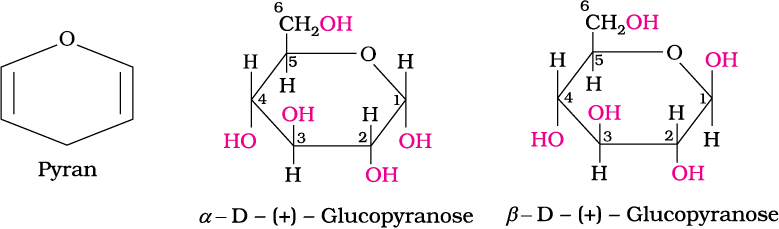
3. Glucose is found to exist in two different crystalline forms which are named as α and β. The α-form of glucose (m.p. 419 K) is obtained by crystallisation from concentrated solution of glucose at 303 K while the β-form (m.p. 423 K) is obtained by crystallisation from hot and saturated aqueous solution at 371 K.
This behaviour could not be explained by the open chain structure (I) for glucose. It was proposed that one of the —OH groups may add to the —CHO group and form a cyclic hemiacetal structure. It was found that glucose forms a six-membered ring in which —OH at C-5 is involved in ring formation. This explains the absence of —CHO group and also existence of glucose in two forms as shown below. These two cyclic forms exist in equilibrium with open chain structure.
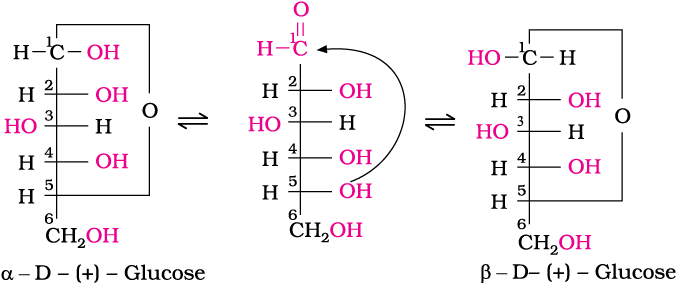
The two cyclic hemiacetal forms of glucose differ only in the configuration of the hydroxyl group at C1, called anomeric carbon
(the aldehyde carbon before cyclisation). Such isomers, i.e., α-form and β-form, are called anomers. The six membered cyclic structure of glucose is called pyranose structure (α– or β–), in analogy with pyran. Pyran is a cyclic organic compound with one oxygen atom and five carbon atoms in the ring. The cyclic structure of glucose is more correctly represented by Haworth structure as given below.