There are various types of hybridisation involving s, p and d orbitals. The different types of hybridisation are as under:
(I) sp hybridisation: This type of hybridisation involves the mixing of one s and one p orbital resulting in the formation of two equivalent sp hybrid orbitals. The suitable orbitals for sp hybridisation are s and pz, if the hybrid orbitals are to lie along the z-axis. Each sp hybrid orbitals has 50% s-character and 50% p-character. Such a molecule in which the central atom is sp-hybridised and linked directly to two other central atoms possesses linear geometry. This type of hybridisation is also known as diagonal hybridisation.
The two sp hybrids point in the opposite direction along the z-axis with projecting positive lobes and very small negative lobes, which provides more effective overlapping resulting in the formation of stronger bonds.
Example of molecule having sp hybridisation
BeCl2: The ground state electronic configuration of Be is 1s22s2. In the exited state one of the 2s-electrons is promoted to vacant 2p orbital to account for its bivalency. One 2s and one 2p-orbital gets hybridised to form two sp hybridised orbitals. These two sp hybrid orbitals are oriented in opposite direction forming an angle of 180°. Each of
the sp hybridised orbital overlaps with the 2p-orbital of chlorine axially and form two Be-Cl sigma bonds. This is shown in Fig. 4.10.
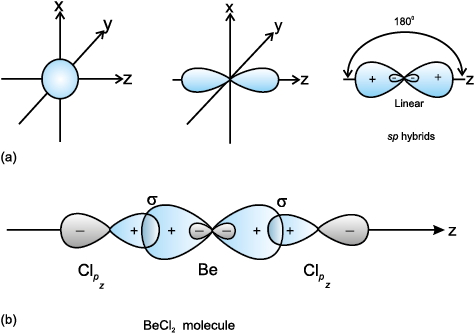
Fig.4.10 (a) Formation of sp hybrids from s and p orbitals; (b) Formation of the linear BeCl2 molecule
(II) sp2 hybridisation : In this hybridisation there is involvement of one s and two
p-orbitals in order to form three equivalent sp2 hybridised orbitals. For example, in BCl3 molecule, the ground state electronic configuration of central boron atom is 1s22s22p1. In the excited state, one of the 2s electrons is promoted to vacant 2p orbital as a result boron has three unpaired electrons. These three orbitals (one 2s and two 2p) hybridise to form three sp2 hybrid orbitals. The three hybrid orbitals so formed are oriented in a trigonal planar arrangement and overlap with 2p orbitals of chlorine to form three B-Cl bonds. Therefore, in BCl3 (Fig. 4.11), the geometry is trigonal planar with ClBCl bond angle of 120°.
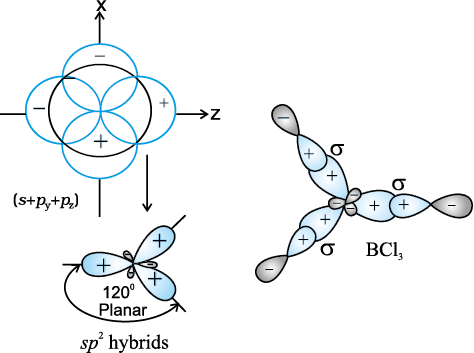
Fig.4.11 Formation of sp2 hybrids and the BCl3 molecule
(III) sp3 hybridisation: This type of hybridisation can be explained by taking the example of CH4 molecule in which there is mixing of one s-orbital and three p-orbitals of the valence shell to form four sp3 hybrid orbital of equivalent energies and shape. There is 25% s-character and 75% p-character in each sp3 hybrid orbital. The four sp3 hybrid orbitals so formed are directed towards the four corners of the tetrahedron. The angle between sp3 hybrid orbital is 109.5° as shown in Fig. 4.12.
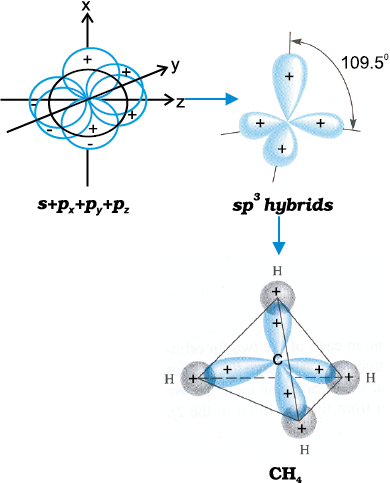
Fig.4.12 Formation of sp3 hybrids by the combination of s , px , py and pz atomic orbitals of carbon and the formation of CH4 molecule
The structure of NH3 and H2O molecules can also be explained with the help of sp3 hybridisation. In NH3, the valence shell (outer) electronic configuration of nitrogen in the ground state is 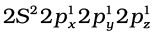 having three unpaired electrons in the sp3 hybrid orbitals and a lone pair of electrons is present in the fourth one. These three hybrid orbitals overlap with 1s orbitals of hydrogen atoms to form three N–H sigma bonds. We know that the force of repulsion between a lone pair and a bond pair is more than the force of repulsion between two bond pairs of electrons. The molecule thus gets distorted and the bond angle is reduced to 107° from 109.5°. The geometry of such a molecule will be pyramidal as shown in Fig. 4.13.
having three unpaired electrons in the sp3 hybrid orbitals and a lone pair of electrons is present in the fourth one. These three hybrid orbitals overlap with 1s orbitals of hydrogen atoms to form three N–H sigma bonds. We know that the force of repulsion between a lone pair and a bond pair is more than the force of repulsion between two bond pairs of electrons. The molecule thus gets distorted and the bond angle is reduced to 107° from 109.5°. The geometry of such a molecule will be pyramidal as shown in Fig. 4.13.
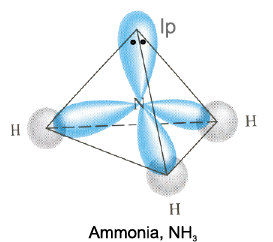
Fig.4.13 Formation of NH3 molecule
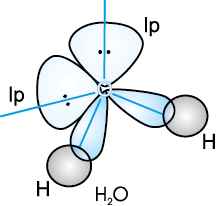
In case of H2O molecule, the four oxygen orbitals (one 2s and three 2p) undergo sp3 hybridisation forming four sp3 hybrid orbitals out of which two contain one electron each and the other two contain a pair of electrons. These four sp3 hybrid orbitals acquire a tetrahedral geometry, with two corners occupied by hydrogen atoms while the other two by the lone pairs. The bond angle in this case is reduced to 104.5° from 109.5° (Fig. 4.14) and the molecule thus acquires a V-shape or angular geometry.