Our theoritical model of gases corresponds very well with the experimental observations. Difficulty arises when we try to test how far the relation pV = nRT reproduce actual pressure-volume-temperature relationship
of gases. To test this point we plot pV vs p plot of gases because at constant temperature, pV will be constant (Boyle’s law) and pV vs p graph at all pressures will be a straight line parallel to x-axis. Fig. 5.10 shows such a plot constructed from actual data for several gases at 273 K.
It can be seen easily that at constant temperature pV vs p plot for real gases is not a straight line. There is a significant deviation from ideal behaviour. Two types of curves are seen. In the curves for dihydrogen and helium, as the pressure increases the value of pV also increases. The second type of plot is seen in the case of other gases like carbon monoxide and methane. In these plots first there is a negative deviation from ideal behaviour, the pV value decreases with increase in pressure and reaches to a minimum value characteristic of a gas. After that pV value starts increasing. The curve then crosses the line for ideal gas and after that shows positive deviation continuously. It is thus, found that real gases do not follow ideal gas equation perfectly under all conditions.
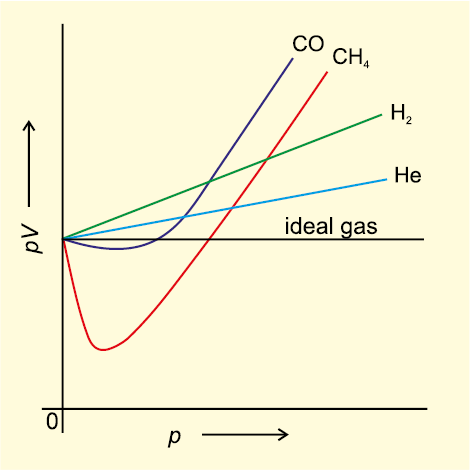
Fig. 5.10 Plot of pV vs p for real gas and ideal gas
Deviation from ideal behaviour also becomes apparent when pressure vs volume plot is drawn. The pressure vs volume plot of experimental data (real gas) and that theoretically calculated from Boyle’s law (ideal gas) should coincide. Fig 5.11 shows these plots. It is apparent that at very high pressure the measured volume is more than the calculated volume. At low pressures, measured and calculated volumes approach each other.
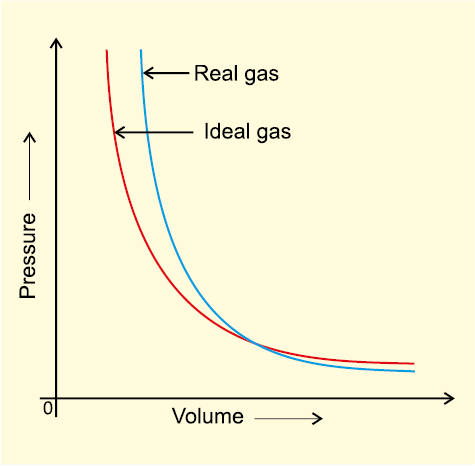
Fig. 5.11 Plot of pressure vs volume for real gas and ideal gas.
It is found that real gases do not follow, Boyle’s law, Charles law and Avogadro law perfectly under all conditions. Now two questions arise.
(i) Why do gases deviate from the ideal behaviour?
(ii) What are the conditions under which gases deviate from ideality?
We get the answer of the first question if we look into postulates of kinetic theory once again. We find that two assumptions of the kinetic theory do not hold good. These are
(a) There is no force of attraction between the molecules of a gas.
(b) Volume of the molecules of a gas is negligibly small in comparison to the space occupied by the gas.
If assumption (a) is correct, the gas will never liquify. However, we know that gases do liquify when cooled and compressed. Also, liquids formed are very difficult to compress. This means that forces of repulsion are powerful enough and prevent squashing of molecules in tiny volume. If assumption (b) is correct, the pressure vs volume graph of experimental data (real gas) and that theoritically calculated from Boyles law (ideal gas) should coincide.
Real gases show deviations from ideal gas law because molecules interact with each other. At high pressures molecules of gases are very close to each other. Molecular interactions start operating. At high pressure, molecules do not strike the walls of the container with full impact because these are dragged back by other molecules due to molecular attractive forces. This affects the pressure exerted by the molecules on the walls of the container. Thus, the pressure exerted by the gas is lower than the pressure exerted by the ideal gas.
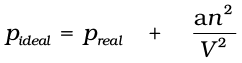 (5.30)
(5.30)
Here, a is a constant.
Repulsive forces also become significant. Repulsive interactions are short-range interactions and are significant when molecules are almost in contact. This is the situation at high pressure. The repulsive forces cause the molecules to behave as small but impenetrable spheres. The volume occupied by the molecules also becomes significant because instead of moving in volume V, these are now restricted to volume (V–nb) where nb is approximately the total volume occupied by the molecules themselves. Here, b is a constant. Having taken into account the corrections for pressure and volume, we can rewrite equation (5.17) as
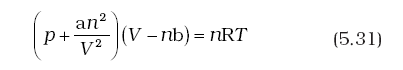
Equation (5.31) is known as van der Waals equation. In this equation n is number of moles of the gas. Constants a and b are called van der Waals constants and their value depends on the characteristic of a gas. Value of ‘a’ is measure of magnitude of intermolecular attractive forces within the gas and is independent of temperature and pressure.
Also, at very low temperature, intermolecular forces become significant. As the molecules travel with low average speed, these can be captured by one another due to attractive forces. Real gases show ideal behaviour when conditions of temperature and pressure are such that the intermolecular forces are practically negligible. The real gases show ideal behaviour when pressure approaches zero.
The deviation from ideal behaviour can be measured in terms of compressibility factor Z, which is the ratio of product pV and nRT. Mathematically
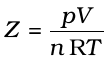 (5.32)
(5.32)
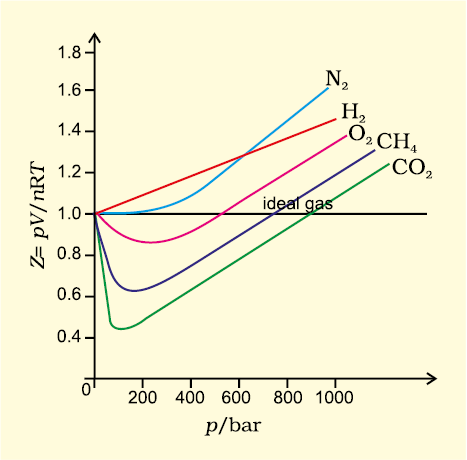
For ideal gas Z = 1 at all temperatures and pressures because pV = n RT. The graph of Z vs p will be a straight line parallel to pressure axis (Fig. 5.12, page 152). For gases which deviate from ideality, value of Z deviates from unity. At very low pressures all gases shown have Z ≈1 and behave as ideal gas. At high pressure all the gases have Z > 1. These are more difficult to compress. At intermediate pressures, most gases have Z < 1. Thus gases show ideal behaviour when the volume occupied is large so that the volume of the molecules can be neglected in comparison to it. In other words, the behaviour of the gas becomes more ideal when pressure is very low. Upto what pressure a gas will follow the ideal gas law, depends upon nature of the gas and its temperature. The temperature at which a real gas obeys ideal gas law over an appreciable range of pressure is called Boyle temperature or Boyle point. Boyle point of a gas depends upon its nature. Above their Boyle point, real gases show positive deviations from ideality and Z values are greater than one. The forces of attraction between the molecules are very feeble. Below Boyle temperature real gases first show decrease in Z value with increasing pressure, which reaches a minimum value. On further increase in pressure, the value of Z increases continuously. Above explanation shows that at low pressure and high temperature gases show ideal behaviour. These conditions are different for different gases.
More insight is obtained in the significance of Z if we note the following derivation
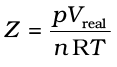 (5.33)
(5.33)
If the gas shows ideal behaviour then
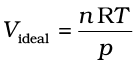 . On putting this value of
. On putting this value of 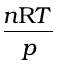 in equation (5.33) we have
in equation (5.33) we have 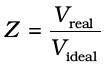 (5.34)
(5.34)
From equation (5.34) we can see that compressibility factor is the ratio of actual molar volume of a gas to the molar volume of it, if it were an ideal gas at that temperature and pressure.
In the following sections we will see that it is not possible to distinguish between gaseous state and liquid state and that liquids may be considered as continuation of gas phase into a region of small volumes and very high molecular attraction. We will also see how we can use isotherms of gases for predicting the conditions for liquifaction of gases.