EXERCISES
Question 13.10:-
Why is benzene extraordinarily stable though it contains three double bonds?
NEETprep Answer
Question 13.11:-
What are the necessary conditions for any system to be aromatic?
NEETprep Answer
Question 13.12:-
Explain why the following systems are not aromatic?
(i) 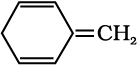
(ii) 
(iii) 
Question 13.13:-
How will you convert benzene into
(i) p-nitrobromobenzene
(ii) m- nitrochlorobenzene
(iii) p - nitrotoluene
(iv) acetophenone?
NEETprep Answer
Question 13.14:-
In the alkane H3C – CH2 – C(CH3)2 – CH2 – CH(CH3)2, identify 1°,2°,3° carbon atoms, and give the number of H atoms bonded to each one of these.
NEETprep Answer
Question 13.15:-
What effect does branching of an alkane chain has on its boiling point?
NEETprep Answer
Question 13.16:-
Addition of HBr to propene yields 2-bromopropane, while in the presence of benzoyl peroxide, the same reaction yields 1-bromopropane. Explain and give mechanisms.
NEETprep Answer
13.17 Write down the products of ozonolysis of 1,2-dimethyl benzene (o-xylene). How does the result support the Kekulé structure for benzene?
NEETprep Answer
Question13.18:-
Arrange benzene, n-hexane and ethyne in decreasing order of acidic behaviour. Also, give a reason for this behaviour.
NEETprep Answer
Question 13.19:-
Why does benzene undergo electrophilic substitution reactions easily and nucleophilic substitutions with difficulty?
NEETprep Answer
Question 13.20:-
How would you convert the following compounds into benzene?
(i) Ethyne
(ii) Ethene
(iii) Hexane
NEETprep Answer
© 2026 GoodEd Technologies Pvt. Ltd.