Type of bonds that stabilize a tertiary structure
of a protein include all the following except:
1.
van der Waal’s interactions
2.
Hydrophobic interactions
3.
Disulfide linkage
4.
Covalent bonds
of a protein include all the following except:
Protein molecules that assist in the proper
folding of other proteins are known as:
1. Ubiquitins
2. Chaperonins
3. Prions
4. Calmodulins
A ribosome inhibiting secondary metabolite
protein found in the castor plant is:
1. Ricin
2. Abrin
3. Concanvalin A
4. Vinblastin
When an enzyme-substrate reaction tends
toward zero order, the only way to make a
reaction speed up is to:
1. Add more substrate
2. Add more enzyme
3. Increase the temperature
4. Increase the pressure in the medium
The Glucose transpoter protein present in the
baso-lateral surface of the intestinal epithelial
cells is:
1. GLUT 1
2. GLUT 2
3. GLUT 3
4. GLUT 4
The plot represents the relationship between
substrate concentration and velocity for a
single enzyme in the absence (curve x) and
presence (curve y) of a compound that binds
allosterically to the enzyme. The allosteric
compound is:
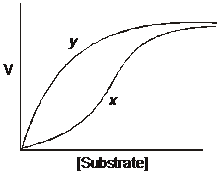
1. a competitive inhibitor.
2. a noncompetitive inhibitor.
3. an irreversible inhibitor.
4. an activator.
The metal ion that acts as a co-factor for both alcohol dehydrogenase and carbonic anhydrase is:
| 1. | Magnesium | 2. | Iron |
| 3. | Zinc | 4. | Nickel |

To unlock all the explanations of 38 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 38 chapters you need to be enrolled in MasterClass Course.
The living state can be best described as a:
1. non equilibrium steady state
2. non equilibrium non steady state
3. equilibrium non steady state
4. equilibrium steady state

To unlock all the explanations of 38 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 38 chapters you need to be enrolled in MasterClass Course.
The blood concentration of glucose in a normal healthy individual is:
| 1. 3.5 – 4.0 mM | 2. 4.0 – 4.5 mM |
| 3. 4.2 – 6.1 mM | 4. 5.0 – 5.5 mM |

To unlock all the explanations of 38 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 38 chapters you need to be enrolled in MasterClass Course.
All the following assumptions apply to Michaelis-Menten kinetic analyses of enzyme action EXCEPT:
1. the total enzyme concentration studied at each substrate concentration is fixed in analysis of enzyme kinetics.
2. formation of enzyme-substrate complex does not appreciably decrease the concentration of substrate.
3. Km decreases with competitive inhibition.
4. maximal velocity is reached when the enzyme-substrate complex is equal to the total concentration of enzyme present.

To unlock all the explanations of 38 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 38 chapters you need to be enrolled in MasterClass Course.






