With respect to the conformers of ethane, which of the following statements is true?
(1) Bond angle remains same but bond length changes
(2) Bond angle changes but bond length remains same
(3) Both bond angle and bond length change
(4) Both bond angles and bond length remain same
The IUPAC name of the compound

1. 3-Keto-2-methylhex-4-enal
2. 5-Formylhex-2-en-3-one
3. 5-Methyl-4-oxohex-2-en-5-al
4. 3-Keto-2-methylhex-5-enal
The correct order of acidity among the following is:
1. CH2=CH2 >CH≡CH > CH3C≡CH > CH3-CH3
2. CH≡CH > CH3-C≡CH > CH2=CH2 >CH3-CH3
3. CH≡CH > CH2=CH2 > CH3-C≡CH > CH3-CH3
4. CH3-CH3 > CH2=CH2 > CH3-C≡CH > CH≡CH
The most suitable method of separation of 1:1 mixture of ortho and para-nitrophenols is
1. sublimation
2. chromatography
3. crystallisation
4. steam distillation
In pyrrole,
The electron density is maximum on
1. 2 and 3 2. 3 and 4
3. 2 and 4 4. 2 and 5
Which among the given molecules can exhibit tautomerisrn?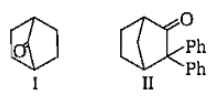
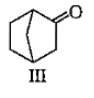
1. III Only
2. Both I and III
3. Both Iand II
4. Both II and III
The correct order of strengths of the carboxylic acids :-
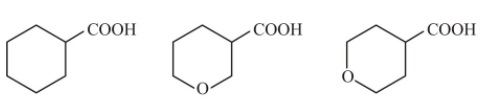
(a) I>II>III
(b) II>III>I
(c) III>II>I
(d) lI>I>IlI
The correct statement regarding the comparison of staggered and eclipsed conformations of ethane is:
| 1. | The eclipsed conformation of ethane is more stable than staggered conformation because eclipsed conformation has no torsional strain |
| 2. | The eclipsed conformation of ethane is more stable than staggered conformation even though the eclipsed conformation has a torsional strain |
| 3. | The staggered conformation of ethane is more stable than the eclipsed conformation because staggered conformation has no torsional strain |
| 4. | The staggered conformation of ethane is less stable than eclipsed conformation because staggered conformation has torsional strain |
In Duma’s method for estimation of nitrogen, 0.25g of an organic compound gave 40 mL
of nitrogen collected at 300 K temperature and 725 mm pressure. If the aqueous tension
at 300 K is 25 mm, the percentage of nitrogen in the compound is
1. 17.36
2. 18.20..
3. 16.76
4. 15.76
The compound that gives the most stable carbonium ion after C- Cl bond ionisation among the following is-
| 1. |  |
2. |  |
| 3. |  |
4. |  |







