A refrigerator works between 4°C and 30°C. It is required to remove 600 calories of heat every second in order to keep the temperature of the refrigerated space constant. The power required is (Take, 1 cal = 4.2 Joules)
1. 23.65 W
2. 236.5 W
3. 2365 W
4. 2.365 W
1. 23.65 W
Figure below shows two paths that may be taken by a gas to go from a state \(A\) to a state \(C.\) In process \(AB,\) \(400\text{ J}\) of heat is added to the system and in process \(BC,\) \(100\text{ J}\) of heat is added to the system. The heat absorbed by the system in the process \(AC\) will be-
1. \(380\text{ J}\)
2. \(500\text{ J}\)
3. \(460\text{ J}\)
4. \(300\text{ J}\)
One mole of an ideal diatomic gas undergoes a transition from A to B along a path AB as shown in the figure.
The change in internal energy of the gas during the transition is
1. 20 kJ
2. -20 kJ
3. 20 J
4. -12 kJ
The coefficient of performance of a refrigerator is 5. If the temperature inside freezer is -20°C, the temperature of the surroundings to which it rejects heat is -
1. 31°C
2. 41°C
3. 11°C
4. 21°C
A thermodynamic system is taken through the cycle ABCD as shown in figure. Heat rejected by the gas during the cycle is
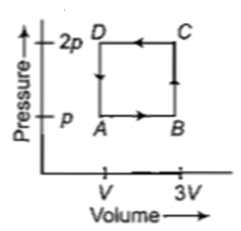
1. 2 pV
2. 4 pV
3.
4. pV
One mole of an ideal gas from an initial state A undergoes via two processes. It first undergoes isothermal expansion from volume V to 3V and then its volume is reduced from 3V to V at constant pressure. The correct P-V diagram representing the two processes is -
1. 
2. 
3. 
4. 
An ideal gas goes from state \(A\) to state \(B\) via three different processes, as indicated in the \(P\text-V\) diagram. If \(Q_1,Q_2,Q_3\) indicates the heat absorbed by the gas along the three processes and \(\Delta U_1, \Delta U_2, \Delta U_3\) indicates the change in internal energy along the three processes respectively, then:

| 1. | \({Q}_1>{Q}_2>{Q}_3 \) and \(\Delta {U}_1=\Delta {U}_2=\Delta {U}_3\) |
| 2. | \({Q}_3>{Q}_2>{Q}_1\) and \(\Delta {U}_1=\Delta {U}_2=\Delta {U}_3\) |
| 3. | \({Q}_1={Q}_2={Q}_3\) and \(\Delta {U}_1>\Delta {U}_2>\Delta {U}_3\) |
| 4. | \({Q}_3>{Q}_2>{Q}_1\) and \(\Delta {U}_1>\Delta {U}_2>\Delta {U}_3\) |
A mass of diatomic gas (=1.4) at a pressure of 2 atm is compressed adiabatically so that its temperature rise from to The pressure of the gas is final state is-
(1) 28 atm
(2) 68.7 atm
(3) 256 atm
(4) 8 atm
In thermodynamic processes, which of the following statements is not true?
| 1. | In an adiabatic process, the system is insulated from the surroundings. |
| 2. | In an isochoric process, the pressure remains constant. |
| 3. | In an isothermal process, the temperature remains constant. |
| 4. | In an adiabatic process, \(P V^\gamma\) = constant. |
If Q, E and W denote respectively the heat added, change in internal energy and the work done in a closed cyclic process, then
1. W=0
2. Q=W=0
3. E=0
4. Q=0








