One mole of an ideal diatomic gas undergoes a transition from A to B along a path AB as shown in the figure.

The change in internal energy of the gas during the transition is
(1) 20 kJ
(2) -20 kJ
(3) 20 J
(4) -12 kJ

(1) 20 kJ
An ideal gas is compressed to half its initial volume by means of several processes.
Which of the following processes results in the maximum work being done on the gas?
1. Adiabatic
2. Isobaric
3. Isochoric
4. Isothermal
The coefficient of performance of a refrigerator is 5. If the temperature inside freezer is -20°C, the temperature of the surroundings to which it rejects heat is -
1. 31°C
2. 41°C
3. 11°C
4. 21°C
A thermodynamic system undergoes cyclic process ABCDA as shown in figure. The work done by the system in the cycle is
(1) ρoVo
(2) 2ρoVo
(3) ρoVo/2
(4)zero
A gas is taken through the cycle A→B→C→A, as shown. What is the net work done by the gas?
(1)2000J
(2)1000J
(3)Zero
(4)-2000J
During an adiabatic process, the pressure of a gas is found to be proportional to the cube of its temperature. The ratio of CP/CV for the gas is equal to:
| 1. | 4/3 | 2. | 2 |
| 3. | 5/3 | 4. | 3/2 |
A thermodynamic system is taken through the cycle ABCD as shown in figure. Heat rejected by the gas during the cycle is
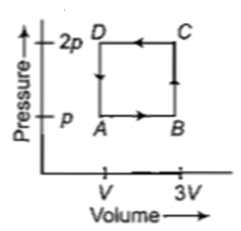
(a)2 pV (b)4 pV
(c) (d)pV
One mole of an ideal gas from an initial state A undergoes via two processes. It first undergoes isothermal expansion from volume V to 3V and then its volume is reduced from 3V to V at constant pressure. The correct P-V diagram representing the two processes is -
An ideal gas goes from state \(A\) to state \(B\) via three different processes, as indicated in the \(P\text-V\) diagram. If \(Q_1,Q_2,Q_3\) indicates the heat absorbed by the gas along the three processes and \(\Delta U_1, \Delta U_2, \Delta U_3\) indicates the change in internal energy along the three processes respectively, then:

| 1. | \({Q}_1>{Q}_2>{Q}_3 \) and \(\Delta {U}_1=\Delta {U}_2=\Delta {U}_3\) |
| 2. | \({Q}_3>{Q}_2>{Q}_1\) and \(\Delta {U}_1=\Delta {U}_2=\Delta {U}_3\) |
| 3. | \({Q}_1={Q}_2={Q}_3\) and \(\Delta {U}_1>\Delta {U}_2>\Delta {U}_3\) |
| 4. | \({Q}_3>{Q}_2>{Q}_1\) and \(\Delta {U}_1>\Delta {U}_2>\Delta {U}_3\) |
A mass of diatomic gas (=1.4) at a pressure of 2 atm is compressed adiabatically so that its temperature rise from to The pressure of the gas is final state is-
(1) 28 atm
(2) 68.7 atm
(3) 256 atm
(4) 8 atm









