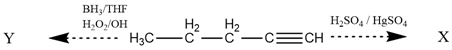
 . Where X and Y are
. Where X and Y are
\(\xleftarrow{\mathrm {BH_3/THF\\{H_2O_2/OH}}}~\mathrm {H_3C-CH_2-CH_2-CH\xrightarrow{H_2SO_4/H_2SO_4}}~\mathrm X\\\)
1. Positional isomers
2. Functional isomers
3. Metamers
4. Tautomers
 . Where X and Y are
. Where X and Y are\(\xleftarrow{\mathrm {BH_3/THF\\{H_2O_2/OH}}}~\mathrm {H_3C-CH_2-CH_2-CH\xrightarrow{H_2SO_4/H_2SO_4}}~\mathrm X\\\)
The property by virtue of which a compound can turn the plane of polarization of light is known as:
1. Photolysis
2. Phosphorescence
3. Optical activity
4. Polarization
The molecular formula of a saturated compound is C2H4Br2. This formula permits the existence of:
1. Functional isomers
2. Optical isomers
3. Positional isomers
4. Cis-trans isomers
Which of the following may exist as enantiomers?
| 1. |  |
| 2. | \(\mathrm{CH}_2=\mathrm{CHCH}_2 \mathrm{CH}_2 \mathrm{CH}_3 \) |
| 3. |  |
| 4. |  |
The total number of isomers could be obtained from the alkane, C6H14 is-
1. Four
2. Five
3. Six
4. Seven
\(\mathrm{CH_3-CHCl-CH_2-CH_3}\) has a chiral center. Which one of the following represents its R-configuration?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The number of primary carbon atoms in the following compound are:

| 1. | 6 | 2. | 2 |
| 3. | 4 | 4. | 3 |
4-methylpent-2-ene is achiral because it has:
1. a centre of symmetry
2. a plane of symmetry
3. symmetry at C 4 carbon
4. both centre and a plane of symmetry
The (R)- and (S)-enantiomers of an optically active compound differ in
1. their solubility in a chiral solvent
2. their reactivity with a chiral reagent
3. their optical rotation of plane polarised light
4. their melting points
The cylindrical shape of an alkyne is due to the presence of-
1. Three sigma C-C bonds
2. Three π C-C bonds
3. Two sigma C-C and one π C-C bonds
4. One sigma C-C and two π C-C bonds






