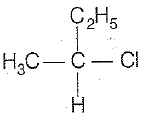
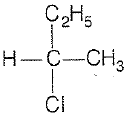
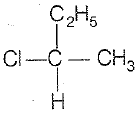
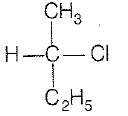
CH3-CHCl-CH2-CH3 has a chiral center. Which one of the following represents its R-configuration?
1. 
2. 
3. 
4. 




The number of primary carbon atoms in the following compound are:
| 1. | 6 | 2. | 2 |
| 3. | 4 | 4. | 3 |
4-methylpent-2-ene is achiral because it has:
1. a centre of symmetry
2. a plane of symmetry
3. symmetry at C 4 carbon
4. both centre and a plane of symmetry
The (R)- and (S)-enantiomers of an optically active compound differ in
1. their solubility in a chiral solvent
2. their reactivity with a chiral reagent
3. their optical rotation of plane polarised light
4. their melting points
The cylindrical shape of an alkyne is due to the presence of-
1. Three sigma C-C bonds
2. Three π C-C bonds
3. Two sigma C-C and one π C-C bonds
4. One sigma C-C and two π C-C bonds
What is decreasing order of basicity of 1°, 2° and 3° ethyl amines and ammonia in aqueous medium?
1. NH3>C2H5NH2>(C2H5)2NH>(C2H5)3N
2. (C2H5)3N>(C2H5)2NH>C2H5NH2> NH3
3. (C2H5)3NH>C2H5NH2>(C2H5)3N>NH3
4. (C2H5)2NH>(C2H5)3N>C2H5NH2>NH3
The shortest C-C bond distance is found in
1. Diamond
2. Ethane
3. Benzene
4. Acetylene
Which one of the following has the shortest carbon-carbon bond length?
1. Benzene
2. Ethene
3. Ethyne
4. Ethane
Which of the following represents the correct order of acidity in the given compounds?
1. FCOOH > COOH > BrCOOH > ClCOOH
2. BrCOOH > ClCOOH > FCOOH > COOH
3. FCOOH > ClCOOH > BrCOOH > COOH
4. COOH > BrCOOH > ClCOOH > FCOOH
Among the following, the strongest acid is:
| 1. | CH3COOH | 2. | CH2ClCH2COOH |
| 3. | CH2ClCOOH | 4. | CH3CH2COOH |







