The correct statement regarding the comparison of staggered and eclipsed conformations of ethane is:
| 1. | The eclipsed conformation of ethane is more stable than staggered conformation because eclipsed conformation has no torsional strain. |
| 2. | The eclipsed conformation of ethane is more stable than staggered conformation even though the eclipsed conformation has a torsional strain. |
| 3. | The staggered conformation of ethane is more stable than eclipsed conformation because staggered conformation has no torsional strain. |
| 4. | The staggered conformation of ethane is less stable than eclipsed conformation because staggered conformation has a torsional strain. |
An unsaturated hydrocarbon 'A' reacts with two molecules of H2 and upon reductive ozonolysis A gives butane-1,4-dial, ethanal, and propanone.
The IUPAC name of A is:
1. 2-Methylocta-2,6-diene
2. 2-Methylocta-1,5-diene
3. 3-Methylocta-2,6-diene
4. 2-Methylocta-1,6-diene
The most suitable reagent for the following conversion is-
| 1. | Hg2+/ H+, H2O | 2. | Na/liquid NH3 |
| 3. | H2, Pd/C, quinoline | 4. | Zn/HCl |
Product \(Z\) in the above-mentioned reaction is:
1. \(\mathrm{CH}_3-\left(\mathrm{CH}_2\right)_3-\mathrm{O}-\mathrm{CH}_2 \mathrm{CH}_3\)
2. \(\left(\mathrm{CH}_3\right)_2 \mathrm{CH}-\mathrm{O}-\mathrm{CH}_2 \mathrm{CH}_3\)
3. \(\mathrm{CH}_3\left(\mathrm{CH}_2\right)_4-\mathrm{O}-\mathrm{CH}_3\)
4. \(\mathrm{CH}_3 \mathrm{CH}_2-\mathrm{CH}\left(\mathrm{CH}_3\right)-\mathrm{O}-\mathrm{CH}_2 \mathrm{CH}_3 \)
The correct IUPAC name of the given compound is:
1. 3-Ethyl-4-ethenylheptane
2. 3-Ethyl-4-propylhex-5-ene
3. 3-(1-Ethyl propyl) hex-1-ene
4. 4-Ethyl-3-propylhex-1-ene
The incorrect IUPAC name among the following is:
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
The isobutyl group among the following is:
| 1. | 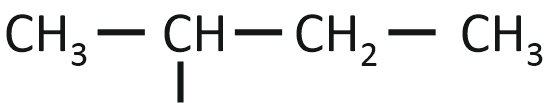 |
| 2. | CH3–CH2–CH2–CH2– |
| 3. | 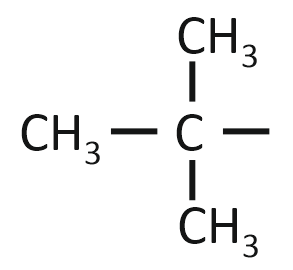 |
| 4. | 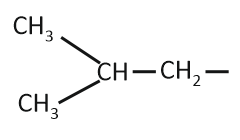 |
If Compound A (C₄H₈) is treated with H₂O/H₂SO₄ and forms an optically inactive C₄H₁₀O, what is the structure of A?
| 1. | CH3CH2CH=CH2 | 2. | CH3CH=CHCH3 |
| 3. | (CH3)2C=CH2 | 4. |
A compound having the shortest carbon-carbon bond length among the following is :
1. Benzene
2. Ethene
3. Ethyne
4. Ethane
Which compound among the following has the highest boiling point?
| 1. | Iso-octane | 2. | n-Octane |
| 3. | 2,2,3,3-Tetramethyl butane | 4. | n-Butane |








