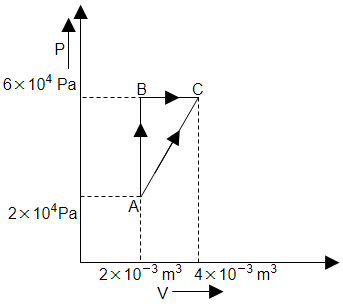
An ideal gas is taken through the cycle \(A\rightarrow B\rightarrow C\rightarrow A\) as shown in the figure below. If the net heat supplied to the gas is \(10~\text{J}\), then the work done by the gas in the process \(B\rightarrow C\) is:

1.
\(-10~\text{J}\)
2.
\(-30~\text{J}\)
3.
\(-15~\text{J}\)
4.
\(-20~\text{J}\)


To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
An ideal gas undergoes a cyclic process ABCA as shown. The heat exchange between the system and the surrounding during the process will be:
| 1. | 10 J | 2. | 5 J |
| 3. | 15 J | 4. | 20 J |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
An ideal gas is taken through the process as shown in the figure. Then:
| 1. | In the process AB, the work done by the system is positive. |
| 2. | In process AB, heat is rejected out of the system. |
| 3. | In the process AB, internal energy increases. |
| 4. | In the process AB, internal energy decreases, and in the process BC, internal energy increases. |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
1 kg of gas does 20 kJ of work and receives 16 kJ of heat when it is expanded between two states. The second kind of expansion can be found between the same initial and final states, which requires a heat input of 9 kJ. The work done by the gas in the second expansion will be:
| 1. | 32 kJ | 2. | 5 kJ |
| 3. | -4 kJ | 4. | 13 kJ |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
| 1. | \(Q_1=Q_2\) |
| 2. | \(W_1=W_2\) |
| 3. | \(Q_1-W_1=Q_2-W_2\) |
| 4. | \(Q_1+W_1=Q_2+W_2\) |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The figure below shows two paths that may be taken by a gas to go from state A to state C. In process AB, \(400~\text{J}\) of heat is added to the system and in process BC, \(100~\text{J}\) of heat is added to the system. The heat absorbed by the system in the process AC will be:
| 1. | \(380~\text{J}\) | 2. | \(500~\text{J}\) |
| 3. | \(460~\text{J}\) | 4. | \(300~\text{J}\) |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
If ΔQ and ΔW represent the heat supplied to the system and
the work done on the system, respectively, then the first law of thermodynamics can be written as: (where ΔU is the internal energy)
1. ΔQ = ΔU + ΔW
2. ΔQ = ΔU – ΔW
3. ΔQ = ΔW – ΔU
4. ΔQ = –ΔU – ΔW

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
Can two isothermal curves cut each other?
| 1. | Never |
| 2. | Yes |
| 3. | They will cut when the temperature is 0°C. |
| 4. | Yes, when the pressure is equal to the critical pressure. |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The latent heat of vaporisation of water is \(2240~\text{J/gm}\). If the work done in the process of expansion of \(1~\text{g}\) is \(168~\text{J}\),
then the increase in internal energy is:
1. \(2408~\text{J}\)
2. \(2240~\text{J}\)
3. \(2072~\text{J}\)
4. \(1904~\text{J}\)

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
1. \(450~\text{K}\)
2. \(375~\text{K}\)
3. \(225~\text{K}\)
4. \(405~\text{K}\)

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.








