A Carnot engine has an efficiency of \(50\%\) when its source is at a temperature \(327^\circ \mathrm{C}\). The temperature of the sink is:
1. \(200^\circ \mathrm{C}\)
2. \(27^\circ \mathrm{C}\)
3. \(15^\circ \mathrm{C}\)
4. \(100^\circ \mathrm{C}\)
| 1. | \(\dfrac{T_1-T_2}{2} \) | 2. | \(T_1 T_2 \) |
| 3. | \(\sqrt{T_1 T_2} \) | 4. | \(\dfrac{T_1+T_2}{2}\) |
An ideal gas heat engine operates in a Carnot cycle between 227ºC and 127ºC. It absorbs 6 kcal at a higher temperature. The amount of heat (in kcal) converted into work is equal to:
1. 4.8
2. 3.5
3. 1.6
4. 1.2
A scientist says that the efficiency of his heat engine which works at source temperature 127ºC and sink temperature 27º C to 26%, then:
1. It is impossible
2. It is possible but less probable
3. It is quite probable
4. Data given is incomplete
The efficiency of a Carnot engine is 50% and the temperature of the sink is 500K. If the temperature of the source is kept constant and its efficiency raised to 60%, then the required temperature of the sink will be:
1. 100 K
2. 600 K
3. 400 K
4. 500 K
The ratio (W/Q) for a Carnot engine is . Now the temperature of the sink is reduced by 62ºC, then this ratio becomes twice. Therefore, the initial temperature of the sink and source are, respectively:
1. 33ºC, 67ºC
2. 37ºC, 99ºC
3. 67ºC, 33ºC
4. 97 K, 37 K
The efficiency of a Carnot engine depends upon:
1. the temperature of the sink only.
2. the temperatures of the source and the sink.
3. the volume of the cylinder of the engine.
4. the temperature of the source only.
Consider a heat engine as shown in the figure. are heat added to and heat taken from respectively, in one cycle of the engine. W is the mechanical work done on the engine.
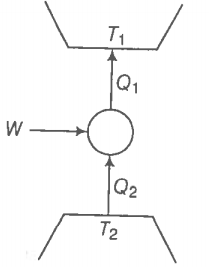
If W > 0, then possibilities are:
Choose the correct alternatives:
1. (b, c)
2. (a, d)
3. (b, d)
4. (a, c)
A refrigerator is to maintain eatables kept inside at \(9^\circ \text{C}.\) If room temperature is \(36^\circ \text{C},\) the coefficient of performance is:
1. \(9.3\)
2. \(12.4\)
3. \(11.2\)
4. \(10.4\)
A steam engine delivers \(5.4\times 10^8\) J of work per minute and extracts \(3.6\times 10^9\) J of heat per minute from its boiler. The efficiency of the engine is:
1. \(15\%\)
2. \(18\%\)
3. \(13\%\)
4. \(11\%\)






