Select Chapter Topics:
Choose the correct option regarding ideal gas:
| 1. | in \(P={\dfrac{m}{M}}RT\), \(m\) is the mass of gas per unit volume. |
| 2. | in \(P={\dfrac{m}{M}}RT\), \(m\) is the mass of one molecule of gas. |
| 3. | in \(P=\dfrac{1}{3} \dfrac{m N}{V} v_{r m s}^2\), \(m\) is the total mass of gas. |
| 4. | in \(v_{r m s}=\sqrt{\dfrac{3 k T}{m}}\), \(m\) is the total mass of the gas. |
Subtopic: Ideal Gas Equation |
52%
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
The volume occupied by the molecules contained in \(4.5~\text{kg}\) water at STP, if the molecular forces vanish away, is:
| 1. | \(5.6~\text{m}^3\) | 2. | \(5.6\times10^{6}~\text{m}^3\) |
| 3. | \(5.6\times10^{3}~\text{m}^3\) | 4. | \(5.6\times10^{-3}~\text{m}^3\) |
Subtopic: Ideal Gas Equation |
Level 3: 35%-60%
NEET - 2022
Hints
Which of the following graphs correctly represents the variation of \(\beta=-\dfrac{1}{V}\dfrac{dV}{dp} \) with \(p \) for an ideal gas at constant temperature?
| 1. | 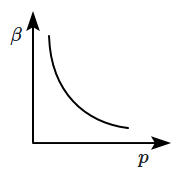 |
2. | 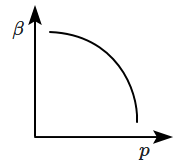 |
| 3. | 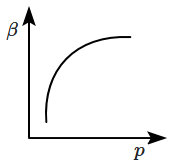 |
4. | 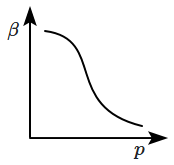 |
Subtopic: Ideal Gas Equation |
75%
Level 2: 60%+
Please attempt this question first.
Hints
Please attempt this question first.
Consider a mole of a sample of hydrogen gas at NTP. Then:
| 1. | the volume of the gas is exactly \(2.24\times10^{-2}\, \text{m}^3.\) |
| 2. | the volume of the gas is approximately \(2.24\times10^{-2}\, \text{m}^3.\) |
| 3. | the gas will be in thermal equilibrium with \(1\) mole of oxygen gas at NTP. |
| 4. | the gas will be in thermodynamic equilibrium with \(1\) mole of oxygen at NTP. |
Subtopic: Ideal Gas Equation |
57%
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
A gas is filled in a vessel at \(27^\circ \text{C}.\) To what temperature should it be heated so that (1/3)rd of the mass of the gas may escape out?
1. \(350\) K
2. \(400\) K
3. \(450\) K
4. \(200\) K
1. \(350\) K
2. \(400\) K
3. \(450\) K
4. \(200\) K
Subtopic: Ideal Gas Equation |
69%
Level 2: 60%+
Please attempt this question first.
Hints
Please attempt this question first.
An inflated rubber balloon contains one mole of an ideal gas, has a pressure \(P,\) volume \(V\) and temperature \(T.\) If the temperature rises to \(1.1T,\) and the volume is increased to \(1.05V,\) the final pressure will be:
| 1. | \(1.1P\) |
| 2. | \(P\) |
| 3. | less than \(P\) |
| 4. | between \(P\) and \(1.1P\) |
Subtopic: Ideal Gas Equation |
71%
Level 2: 60%+
Hints
Unlock IMPORTANT QUESTION
This question was bookmarked by 5 NEET 2025 toppers during their NEETprep journey. Get Target Batch to see this question.
✨ Perfect for quick revision & accuracy boost
Buy Target Batch
Access all premium questions instantly
\(1\) mole of \(\mathrm{H}_{2}\) gas is contained in a box of volume \(V = 1.00 ~\text m^{3}\) at \(T = 300 ~\text{K}\). The gas is heated to a temperature of \(T = 3000~\text{K}\) and the gas gets converted to a gas of hydrogen atoms. The final pressure would be:
(considering all gases to be ideal)
(considering all gases to be ideal)
| 1. | same as the pressure initially. |
| 2. | \(2\) times the pressure initially. |
| 3. | \(10\) times the pressure initially. |
| 4. | \(20\) times the pressure initially. |
Subtopic: Ideal Gas Equation |
53%
Level 3: 35%-60%
Hints
Unlock IMPORTANT QUESTION
This question was bookmarked by 5 NEET 2025 toppers during their NEETprep journey. Get Target Batch to see this question.
✨ Perfect for quick revision & accuracy boost
Buy Target Batch
Access all premium questions instantly
A cylinder containing an ideal gas is in a vertical position and has a piston of mass \(M\) that is able to move up or down without friction (figure). If the temperature is increased,

| 1. | both \(P\) and \(V\) of the gas will change. |
| 2. | only \(P\) will increase according to Charles' law. |
| 3. | \(V\) will change but not \(P.\) |
| 4. | \(P\) will change but not \(V.\) |
Subtopic: Ideal Gas Equation |
Level 3: 35%-60%
Hints
Which of the following diagrams (figure) depicts ideal gas behaviour?
| 1. | (a), (c) | 2. | (a), (d) |
| 3. | (c), (d) | 4. | (a), (b) |
Subtopic: Ideal Gas Equation |
70%
Level 2: 60%+
Hints
An ideal gas equation can be written as \(P = \dfrac{ρRT}{M_{0}}\) where \(\rho\) and \(M_{0}\) are respectively:
| 1. | mass density, the mass of the gas. |
| 2. | number density, molar mass. |
| 3. | mass density, molar mass. |
| 4. | number density, the mass of the gas. |
Subtopic: Ideal Gas Equation |
79%
Level 2: 60%+
NEET - 2020
Hints







