The isobutyl group among the following is:
| 1. | |
| 2. | CH3–CH2–CH2–CH2– |
| 3. | 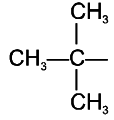 |
| 4. |
What type of orbital overlap results in the formation of the C–H and C–C bonds in ethane?
1.
2.
3. sp – sp and sp – sp
4. p–s and p – p
The alkene among the following that has the smallest heat of hydrogenation is-
1.
2.
3.
4.
The correct order of relative rates of hydrogenation of alkenes is:
1. Ethylene > propene > 2-butene > 2-methyl-2-butene
2. 2-methyl-2-butene > 2-butene > Propene > Ethylene
3. 2-butene > propene > ethylene > 2-methyl-2-butene
4. Propene > 2-butene > ethylene > 2-methyl-2-butene
A compound that gives an optically inactive compound on treatment with H2(2 moles)/ Pt is-
1. 3-Methyl-1-pentyne
2. 4-Methyl-1-hexyne
3. 3-Methyl-1-heptyne
4. None of the above
Which alkene among the following reacts the fastest with H2 under catalytic conditions?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The number of isomeric sodium salt of acid that will be required to obtain neopentane is-
1. 3
2. 1
3. 4
4. 6
undergoes Wurtz reaction to give-
| 1. | Propane + Ethane | 2. | Propane |
| 3. | Propane + Ethane + Butane | 4. | Propane + Butane |
Hydrocarbon (A) reacts with bromine by substitution reaction to form an alkyl bromide B.
B undergoes the Wurtz reaction to give a gaseous hydrocarbon containing less than four carbon atoms.
The formula of (A) is:
1.
2.
3.
4.
Which of the following compounds has the highest melting point?
1.
2.
3.
4.






