Vanillin, used as a flavouring agent, is:
1. An Aliphatic alcohol
2. An Aromatic aldehyde
3. A Hydrocarbon
4. A Carbohydrate
1. Pentane-2,4-dione
2. Pentane-1,4-dione
3. Pentane-2,2-dione
4. Pentane-3,4-dione
1. Diphenylmethanone
2. Benzophenone
3. Dibenzylmethanone
4. Dibenzylketone
Match the common names given in Column I with the IUPAC names given in Column II.
| Column l (Common names) |
Column ll (IUPAC names) |
| A. Cinnamaldehyde | 1. Pentanal |
| B. Acetophenone | 2. Prop-2-enal |
| C. Valeraldehyde | 3. 1-Phenylethanone |
| D. Acrolein | 4. 3-Phenylprop-2-en-al |
Codes:
| A | B | C | D | |
| 1. | 2 | 3 | 4 | 1 |
| 2. | 3 | 1 | 4 | 2 |
| 3. | 1 | 4 | 3 | 2 |
| 4. | 4 | 3 | 1 | 2 |
The correct IUPAC name of the following compound is:

| 1. | 2-Ethylhex-3-en-4-one | 2. | 4-Methylhex-3-en-2-one |
| 3. | 4-Ethylpent-3-en-2-one | 4. | 3-Methylhex -3-en-4-one |
A compound with the molecular formula C5H10 that yields acetone on ozonolysis is:
1. 2-Methyl-2-butene
2. 2-Methyl-1-butene
3. Cyclopentane
4. 3-Methyl-1-butene
What is the structural difference between an aldehyde and a ketone?
1. -H-atom
2. H-atom combined with carbonyl group
3. OH and carbonyl group
4. None of the above
An alkene “A” on reaction with O3 and Zn - H2O gives propanone and ethanal in an equimolar ratio. The addition of HCl to alkene “A” gives “B” as the major product. The structure of product “B” is:
| 1. | 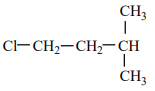 |
2. | 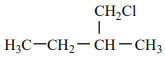 |
| 3. | 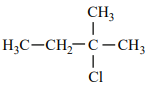 |
4. | 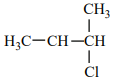 |
Select the correct option based on the statements below:
| Assertion (A): | The geometry of formaldehyde molecule is planar. |
| Reason (R): | Formaldehyde molecule contains sp2 hybridized carbon atom. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | Both (A) and (R) are False. |
The hydrogenation of benzoyl chloride in the presence of Pd and BaSO4 gives:
1. Benzyl alcohol
2. Benzaldehyde
3. Benzoic acid
4. Phenol






