The correct statement regarding the comparison of staggered and eclipsed conformations of ethane is:
| 1. | The eclipsed conformation of ethane is more stable than staggered conformation because eclipsed conformation has no torsional strain. |
| 2. | The eclipsed conformation of ethane is more stable than staggered conformation even though the eclipsed conformation has a torsional strain. |
| 3. | The staggered conformation of ethane is more stable than eclipsed conformation because staggered conformation has no torsional strain. |
| 4. | The staggered conformation of ethane is less stable than eclipsed conformation because staggered conformation has a torsional strain. |
The most suitable reagent for the following conversion is-

1. Hg2+/ H+, H2O
2. Na/liquid NH3
3. H2, Pd/C, quinoline
4. Zn/HCl
Product Z in the above-mentioned reaction is:
1.
2.
3.
4.
The incorrect IUPAC name among the following is:
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Which compound among the following has the highest boiling point?
| 1. | Iso-octane | 2. | n-Octane |
| 3. | 2,2,3,3-Tetramethyl butane | 4. | n-Butane |
Which of the following hydrocarbon fuels has the highest octane rating?
1. Methane
2. Ethane
3. Iso-octane
4. Triptane
undergoes Wurtz reaction to give-
| 1. | Propane + Ethane | 2. | Propane |
| 3. | Propane + Ethane + Butane | 4. | Propane + Butane |
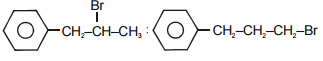
The main product A and B in the above mentioned reaction are respectively-
1.
2.
3. 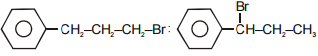
4. 
3-Hexyne can be converted to trans-3-Hexene by the action of:
1. - Pd/
2. Li-Liq.
3. - Pt
4.
The major product in the above-mentioned reaction is:
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |











