Which of the following is the correct representation for intermediate of nucleophilic addition reaction to the given carbonyl compound (A) :
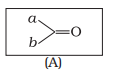
| a. | 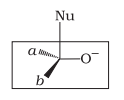 |
| b. | 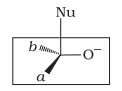 |
| c. | 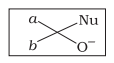 |
| d. | 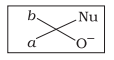 |
Choose the correct option
| 1. | (a, b) | 2. | (b, c) |
| 3. | (c, d) | 4. | (a, d) |
Addition of water to alkynes occurs in acidic medium and in the presence of ions as a catalyst. Which of the following products will be formed on addition of water to but-1-yne under these conditions?
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
The most reactive compound among the following toward nucleophilic addition reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The correct order of increasing acidic strength is:
| 1. | Phenol < Ethanol < Chloroacetic acid < Acetic acid |
| 2. | Ethanol < Phenol < Chloroacetic acid < Acetic acid |
| 3. | Ethanol < Phenol < Acetic acid < Chloroacetic acid |
| 4. | Chloroacetic acid < Acetic acid < Phenol < Ethanol |
Compound can be prepared by the reaction of-
1. Phenol and benzoic acid in the presence of NaOH
2. Phenol and benzoyl chloride in the presence of pyridine
3. Phenol and benzoyl chloride in the presence of
4. Phenol and benzaldehyde in the presence of palladium
The reagent which does not react with both, acetone and benzaldehyde?
1. Sodium hydrogen sulphite
2. Phenyl hydrazine
3. Fehling's solution
4. Grignard reagent
Cannizzaro’s reaction is not given by:
| 1. |  |
2. |  |
| 3. | HCHO | 4. | CH3CHO |
The compound below is treated with a concentrated aqueous KOH solution. The products obtained are:

| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
In the above reaction, the structure of ‘A’ and the type of isomerism shown in the final product are, respectively,:
| 1. | Prop-1-en-2-ol and Metamerism |
| 2. | Prop-1-en-1-ol and Tautomerism |
| 3. | Prop-2-en-2-ol and Geometrical isomerism |
| 4. | Prop-1-en-2-ol and Tautomerism |
Compounds A and C in the following reaction are:
\(\mathrm{\small{{CH}_3 {CHO} \xrightarrow[{(ii) ~H_{2}O}]{(i) ~CH_{3}MgBr}({A}) \stackrel{{H}_2 {SO}_4}{\longrightarrow}({B}) \xrightarrow[]{Hydroboration \ oxidation}(C)}}\)
1. Identical
2. Positional isomers
3. Functional isomers
4. Optical isomers







