An ion Mna+ has the magnetic moment equal to 4.9 B.M. The value of a is:
(1) 3
(2) 4
(3) 2
(4) 5
The maximum wavelength of light that can excite an electron from first to third orbit of hydrogen atom is:
1. 487 nm
2. 170 nm
3. 103 nm
4. 17 nm
The first emission line of Balmer series for H-spectrum has the wave number equal to
(1)
(2)
(3)
(4)
The work function for a metal is 4 eV. To emit a phtoelectron of zero velocity from the surface of the metal, the wavelength of incident light should be:
(1) 2700
(2) 1700
(3) 5900
(4) 3100
Photons of energy of 6 eV are indicated on a potassium surface of work function 2.1 eV. What is the stopping potential?
(1) -6 V
(2) -2.1 V
(3) -3.9 V
(4) -8.1 V
In Bohr's model of the hydrogen atom the ratio between the period of revolution of an electron in the orbit n = 1 to the period of revolution of the electron in the orbit n = 2 is:
(1) 1 : 2
(2) 2 : 1
(3) 1 : 4
(4) 1 : 8
Energy levels A,B,C of a certain atom corresponds to increasing values of energy, i.e., <<. If , and are the wavelengths of radiations corresponding to the transitions C to B, B to A and C to A respectively, which of the following statements is correct?
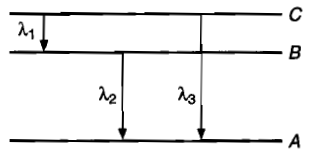
(1) =+
(2) =
(3) ++=0
(4)
The radii of two of the first four Bohr’s orbits of the hydrogen atom are in the ratio 1:4. The energy difference between them may be:
(1) either 12.09eV or 3.4eV
(2) either 2.55eV or 10.2eV
(3) either 13.6eV or 3.4eV
(4) either 3.4eV or 0.85eV
When photons of energy 4.25eV strike the surface of a metal A, the ejected photoelectrons have maximum kinetic energy, (expressed in eV) and de Broglie wavelength , The maximum kinetic energy of photoelectrons liberated from another metal B by photons of energy 4.70V is =−1.50eV. If the de Broglie wavelength of these photoelectrons is =2, then which is not correct?
(1) The work function of A is 2.25eV
(2) The work function of B is 3.70eV
(3) =2.00eV
(4) =0.5eV
The frequency of certain line of the Lyman series of the atomic spectrum of hydrogen satisfies the following conditions:
(i) It is the sum of the frequencies of another Lyman line and a Balmer line.
(ii) It is the sum of the frequencies of a certain line, a Lyman line, and a Paschen line.
(iii)It is the sum of the frequencies of a Lyman and a Paschen line but no Bracket line. To what transition does correspond?
(1)
(2)
(3)
(4)
The number of nodal planes in a -orbital is :
1. 1
2. 2
3. 3
4. zero
If is the Rydberg constant, then the energy of an electron in the ground state of a hydrogen atom is:
1.
2.
3.
4.
The number of electrons in an atom with atomic number 105 having (n+1) = 8 are:
1. 30
2. 17
3. 15
4. Unpredictable
Non-directional orbital is:
1. 3s
2. 4f
3. 4d
4. 4p
Out of the first 100 elements, number of elements having electrons in 3d-orbitals are :
1. 80
2. 10
3. 100
4. 60
Of the following transition in hydrogen atom, the one which givens an absorption line of lowest frequency is:
1. n = 1 to n =2
2. n = 3 to n = 8
3. n = 2 to n = 1
4. n = 8 to n = 3
The angular momentum of an electron in 2 p-orbital is:
(1)
(2)
(3)
(4) None of these
Which are in the ascending order of wavelength?
(1) ...lines in Balmer series of hydrogen atom
(2) Lyman limit, balmer limit, paschen limit in the hydrogen spectrum
(3) Blue, violet, yellow, red colours in solar spectrum
(4) None of the above
Photoelectric emission is observed from a surface for frequency and of the incident radiation (>). If the maximum kinetic energies of the photoelectrons in the two cases are in the ratio 1:k, then the threshold frequency is given by :
(1)
(2)
(3)
(4)
In H atom, the electron is de-excited from fifth shell to first shell. How many different lines may appear in line spectrum?
1. 4
2. 8
3. 10
4. 12