How many formula units are there in the unit cell of sodium chloride?
1. 2
2. 4
3. 6
4. 8
In a compound, atoms of element Y form ccp lattice and those of element X occupy 2/3rd of tetrahedral voids. The formula of compound will be
1.
2.
3.
4.
The edge of a unit cell with fcc structure in Xe crystal is 620 pm. The radius of Xe atom is
1. 219.23 pm
2. 235.16 pm
3. 189.37 pm
4. 319.23 pm
Schottky defect occurs mainly in electrovalent compound where
1. Positive ions have large sizes
2. Negative ions have small sizes
3. Positive and negative ions are of similar size
4. Positive ions are big and negative ions are small
The edge length of fcc unit cell of NaCl is 508 pm. If the radius of cation is 110 pm, then the radius of the anion is :
1. 144 pm
2. 249 pm
3. 288 pm
4. 398 pm
An element (Atomic mass = 50 g/mol) having fcc structure has unit cell edge length 400 pm. The density of element is
1. 5.188 g/cc
2. 10.376 g/cc
3. 2.56 g/cc
4. 1.2 g/cc
In a crystalline solid, anion B are arranged in cubic close packing and cation A are equally distributed between octahedral and tetrahedral voids. If all the octahedral voids are occupied, the formula of the solid is
1.
2.
3.
4.
In a face centred cubic lattice, atom a occupies the face centred positions. If one atom of B is missing from one of the face centred points, the formula of the compound is
1.
2.
3.
4.
A binary solid has a structure with B- ion constituting the lattice and A+ ion occupying 25% tetrahedral holes. The formula of solid is
1.
2.
3.
4.
A solid has a bcc structure. If the distance of closest approach between the two atoms . The edge length of the cell is
1. 200 pm
2.
3. 142.2 pm
4. 400 pm
Analysis shows that an oxide ore of nickel has formula . The percentage of nickel as ions is nearly
1. 2
2. 96
3. 4
4. 98
Perovskite is mineral containing calcium, oxygen and titanium, in which oxygen atoms are at the face centres, calcium atoms are at the corners and titanium atoms at the centre of the cube. Oxidation number of titanium in the mineral is
1. +2
2. +3
3. +4
4. +3/4
If the distance between Na+ and Cl- ions in sodium chloride crystal is X pm, the length of the edge of the unit cell is
1. 4X pm
2. X/4 pm
3. X/2 pm
4. 2X pm
In a crystal A atoms are present at all the corners and face centres while B atoms at all the edge centres and body centre of cubic unit cell. If the atoms from one of the body diagonal are removed then what will be the simplest composition of unit cell?
1.
2.
3.
4.
The number of cation vacancies per mole in NaCl crystal, if it is doped with mole% (ionic salt), are
1.
2.
3.
4.
The arrangement ABC ABC .... is referred to as
1. Octahedral close packing
2. Hexagonal close packing
3. Tetrahedral close packing
4. Cubic close packing
Which of the following adopts normal spinel structure?
1.
2.
3.
4.
The packing efficiency of the two dimensional square unit cell shown below is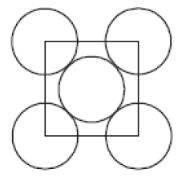
1. 39.27%
2. 68%
3. 74%
4. 78.54%
Which one of the following has Frenkel defect?
1. NaCl
2. AgBr
3. Graphite
4. Diamond
In which operation(s) in the unit cell of sodium chloride, formula of sodium chloride is disturbed?
1. A body diagonal plane is placed
2. A tertrad axis is placed
3. A body diagonal line is placed
4. A rectangular plane is placed