The basic character of methylamines in vapour phase is
(1)
(2)
(3)
(4) None of these
Indicate the correct statement.
(1) is acidic
(2)
(3)
(4)
Which of the following reaction does not yield an amine?
1.
2.
3.
4.
What is the end product in the following sequence of reactions,
(1) Ethyl cyanide
(2) Ethylamine
(3) Methylamine
(4) Acetamide
urea on heating with ethanol gives:
1. urethane
2. urea alcohol
3. ureides
4. none of these
The type of isomerism shown by is:
1. Position
2. Functional
3. Enantiomers
4. Tautomerism
Carbylamine reaction tubes are not thrown into sink, to avoid bad odour, but are treated with conc. HCl to give:
(1)
(2)
(3)
(4)
Which of the following can be used to distinguished acetamide and urea?
1. Fehling's solution
2. Biuret test
3. Hofmann's reaction
4. NaOH solution
Nitroalkane is acidic only towards:
(1)
(2) NaOH
(3) alcohol
(4) liquid
A compound A when reacted with PC and then with ammonia gave B. B when treated with bromine and caustic potash produced C . C on treatment with NaN and HCl at 0 and then boiling produced orthocresol. Compound A is:
(1) o - toluic acid
(2) o - chlorotoluene
(3) o - bromotoluene
(4) m -toluic acid
Benzoyl chloride does not react with:
1. Primary or secondary amines
2. Aliphatic compounds
3. Aromatic compounds
4. Carboxylic acids
The IUPAC name of the compound having formula,
1. 3- aminohydroxy propionic acid
2. 2- amino-propan-3-oic acid
3. amino hydroxy propanoic acid
4. 2-amino-3 hydroxy propanoic acid
The product[A] formed in the reaction;
1. ![]()
2. 
3. ![]()
4. ![]()
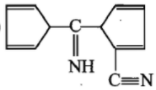
In the reaction, ![]() Product. The Product is :
Product. The Product is :
(1) 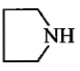
(2) 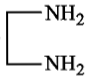
(3) 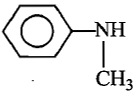
(4) ![]()
Allyl isocyanide contains ........ and ....... bonds.
(1)
(2)
(3)
(4)
How many primary amines are possible for the formula ?
(1) 1
(2) 2
(3) 3
(4) 4
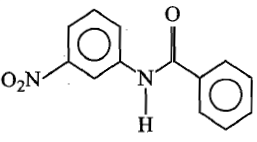
In the following reaction, product X is: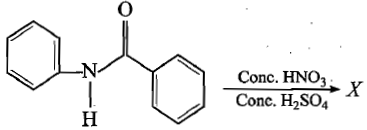
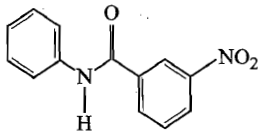
1. 
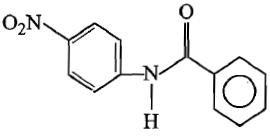
2. 
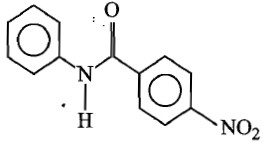
3. 
4. 
Phenol on treatment with dil. at room temperature gives:
| 1. | 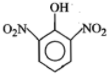 |
2. | 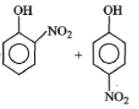 |
| 3. | 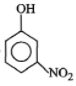 |
4. | 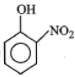 |
During the electrophilic substitution reaction of pyridine, at which position is substitution most likely to occur?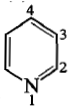
1. Position 2
2. Position 3
3. Position 4
4. Positions 2 and 4
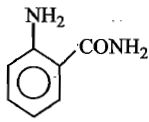
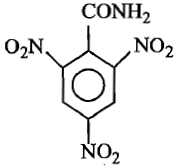
The formula of picramide is:
(1) 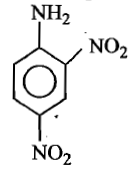
(2)
(3) 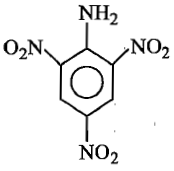
(4) 
The diazonium salt 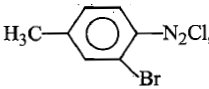 , gives,
, gives,![]() , with:
, with:
(1)
(2)
(3)
(4)
Choose the correct statement from the ones given below for two anilium in: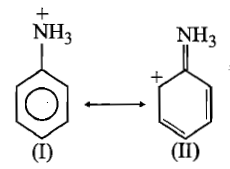
(1) II is not an acceptable canonical structure because carbonium ions are less stable than ammonium ions
(2) II is not an acceptable canonical structure because it is non-aromatic
(3) II is not an acceptable canonical structure because the nitrogen has 10 valence electrons
(4) II is an acceptable canonical structure
A compound with molar mass 180 is acylated with to get a compound with molar mass 390. The number of amino groups present per molecule of the former compound is:
(1) 4
(2) 6
(3) 2
(4) 5