
The IUPAC name of the above mentioned compound is -
1. 1,2,3-Tricyanopropane
2. Propane-1,2,3-tricarbonitrile
3. 1,2,3-Cyanopropane
4. Propane Tricarbylamine
The IUPAC name of the compound

1. 3,3-diethyl-4-methyl-5 (methylethyl)octane
2. 3,3-diethyl-5-isopropyl-4-methyloctane
3. 4-isopropyl-5-methyl-6,6-diethyloctane
4. 6,6-diethyl-4-isopropyl-5-methyloctane
The IUPAC name of the compound is
is
(1) 3,3-diethyl-4-methyl-5-isopropyloctane
(2) 3,3-diethyl-5-isopropyl-4-methyloctane
(3) 4-isopropyl-5-methyl-6,6-diethyloctane
(4) 6,6-diethyl-4-isopropyl-5-methyloctane
Which of the following species exhibits paramagnetism?
| 1. | A carbocation | 2. | A free radical |
| 3. | A carbanion ion | 4. | All of the above |
The structure of cis-bis (propenyl) ethene is:
1. 
2. 
3. 
4. 
The incorrect IUPAC/Common name of  is:
is:
1. Isopropyl benzene
2. Cumene
3. Phenyl isopropane
4. Propan-3-ylbenzene
Number of monochlorinated products (excluding stereo-isomers) obtained from the given reaction are :
| 1. | 4 | 2. | 5 |
| 3. | 6 | 4. | 7 |
How many structural isomers are possible for the compound having molecular formula C3H5Br3?
1. 5
2. 4
3. 6
4. 8
Which of the following acid does not exhibit optical isomerism?
1. Maleic acid
2. -amino acid
3. Lactic acid
4. Tartaric acid
The prefix used for -SH group as per the IUPAC system is-
1. Mercapto
2. Thiol
3. Sulfide
4. None of the above
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
The relative stability order of carbanions CH≡C-, CH3 - and CH2=CH- is ____________
1.
2.
3.
4.
Which of the following is the strongest nucleophile in aprotic solvent?
1. Br-
2. OH-
3. CN-
4. CH3O-
Which of the following is the most stable carbocation
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
The IUPAC name of the following compounds is:

1. 1-Bromocyclohexane carboxylic acid
2. 3-Bromocyclohexanoic acid
3. 3-Bromoheptanoic acid
4. 3-Bromocyclohexanecarboxylic acid
The correct order of basicities of the following compounds is:
(1)
(2)
(3)
(4)
1. 2>1>3>4
2. 1>3>2>4
3. 3>1>2>4
4. 1>2>3>4
The most reactive of these compounds towards sulphonation is
1. Toluene 2. Chlorobenzene
3. Nitrobenzene 4. m-Xylene
How many geometrical isomers are possible of the following?
CH3-CH=CH-CH=CH-CH3
1. 2 2. 3
3. 4 4. 6
Correct IUPAC name of the compound
1. 4-(Ethyl methanolyonxy)phenylpropanoate
2. Ethyl 4-propanoyloxybenzenecarboxylate
3. 4-(1-Oxo-2-oxabutyl)phenylpropanoate
4. 1-(1-Oxo-2-oxbutyl)-4-(1-oxopropoxy)benzene
The IUPAC name of the following compound is
1. 2-(Ethoxycarbonyl)benzoylchloride
2. Ethyl 2-(chlorocarbonyl)benzoate
3. Ethyl 2-(chloromethanoyl)benzoate
4. Methyl 2-(Chlorocarbonyl)benzene carboxylate.
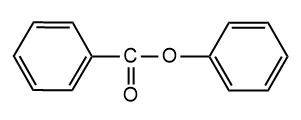 and
and 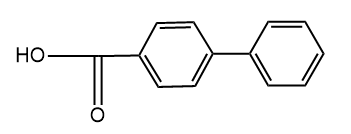 are
are
1. Position isomers
2. Chain isomers
3. Functional isomers
4. Metamers
Which of the following carbocation will undergo rearrangement ?
1. 
2.
The colour of the solution that gets formed by mixing sodium nitroprusside to an alkaline solution of sulfide ions, is-
| 1. | Red | 2. | Blue |
| 3. | Brown | 4. | Purple |
In Kjeldahl's method of estimation of nitrogen, K2SO4 acts as:
| 1. | An oxidizing agent | 2. | A catalytic agent |
| 3. | A hydrolyzing agent | 4. | A boiling point elevator |
The Prussian blue colour obtained during the test of nitrogen by Lassaigne's test is due to the formation of-
| 1. | Fe4[Fe(CN)6]3 | 2. | Na3[Fe(CN)6] |
| 3. | Fe(CN)3 | 4. | Na4[Fe(CN)5NOS] |
A compound that does not give a positive test in Lassaigne’s test for nitrogen is:
1. Urea
2. Hydrazine
3. Azobenzene
4. Phenylhydrazine
The IUPAC name of the below given compound is:
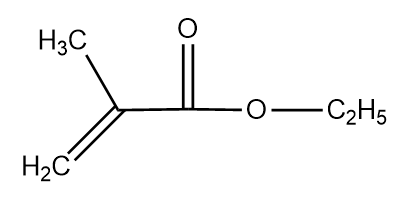
1. Ethyl 2-methylprop-2-enoate
2. Ethyl 2-methylprop-1-enoate
3. 1-Ethoxy 2-methylprop-2-enoate
4. 1-Ethoxy 2-methylprop-2-enal
The IUPAC name of 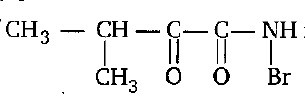 is:
is:
1. (N-Bromo)-2-keto-3-methylbutanamide
2. (Bromo) -2-keto-4-methylbutanamide
3. (N-Bromo)-1, 2-diketo-3-methylbutanamine carboxamide
4. (N-Bromo)-1-keto-2-methylpropane
The reagent that is used to remove and Cl- is -
1. NaOH
2. Pb(NO3)2
3. BaSO4
4. KOH
The silver sulphate solution is used to separate:
1. nitrate and bromide
2. nitrate and chlorate
3. bromide and iodide
4. nitrate and nitrite
Soda extract is prepared by-
1. Fusing soda and mixture of hydrocarbons, and then extracted with water
2. Dissolving NaHCO3 and mixture of hydrocarbons in dil. HCl
3. Boiling Na2CO3 and mixture of hydrocarbons in dil. HCl
4. Boiling Na2CO3 and mixture of hydrocarbons in distilled water
H2C = O behaves as:
1. Nucleophile
2. Electrophile
3. Both (1) and (2)
4. None of the above
Which of the following is strongest nucleophile?
1. Br-
2.
3.
4.
Which of the following is singlet carbene?
1. (CH3)3C+
2. C2H5-CH
3.
4. CH2 =CH-
The number of geometrical isomers in case of a compound with the structure,
CH3-CH=CH-CH=CH-C2H5 are:
1. Four
2. Three
3. Two
4. Five
Lactic acid is:
1. propionic acid
2. β-hydroxypropanoic acid
3. α-hydroxypropanoic acid
4. none of the above
The number of different amines corresponding to the formula C3H9N is:
1. 2
2. 3
3. 4
4. 5
Hydrogen cyanide and hydrogen isocyanide are:
1. tautomers
2. positional isomers
3. metamers
4. chain isomers
The optically active compound among the following is-
1. Isobutyric acid
2. beta-Chloropropionic acid
3. Propionic acid
4. alpha-Chloropropionic acid
The isomeric cis-2-Butene and trans-2-Butene can be distinguished on the basis of:
1. Their colour.
2. Their reduction products.
3. The products they give on ozonolysis..
4. The products they give on addition to bromine.
In the Kjeldahl's method for estimation of nitrogen present in a soil sample, ammonia evolved from 0.75 g of sample neutralised 10 mL of 1 M H2SO4. The percentage of nitrogen in the soil is
1. 37.33 2. 45.33
3. 35.33 4. 43.33
In Duma's method of estimation of nitrogen, 0.35 g of an organic compound gave 55 ml of nitrogen collected at 300 K temperature and 715 mm pressure. The percentage composition of nitrogen in the compound would be:
(Aqueous tension at 300 K = 15 mm)
1. 16.45
2. 27.45
3. 44.45
4. 35.45
The Lassaigne's extract is boiled with a concentration HNO3 while testing for halogens. By doing so it:
1. Helps in the precipitation of AgCl.
2. Increases the solubility product of AgCl.
3. Increases the concentration of NO3- ions.
4. Decomposes Na2S and NaCN, if formed.
Among the given compounds, the most susceptible to nucleophilic attack at the carbonyl group is
1. CH3COOCH3
2. CH3CONH2
3. CH3COOCOCH3
4. CH3COCl
How many stereoisomers does this molecule have?
CH3CH=CHCH2C HBrCH3
1. 4
2. 6
3. 8
4. 2