In bisulphate ion, HSO4-, the formal charge on S atom is
1. +6
2. +4
3. +1
4. +2
Among the following isostructural compounds, which one has highest lattice energy ?
1. LiCl
2. MgO
3. NaCl
4. LiF
A bonded molecule MX3 is T-shaped. The number of non-bonding pairs of electrons is:
1. 0
2. 2
3. 1
4. Can be predicted only if atomic number of M is known
The hybrid state of S in SO3 is similar to that of
1. C in C2H2
2. C in C2H4
3. C in CH4
4. C in CO
In a chemical change from PCl3 PCl5 the hybrid state of P changes from :
1. sp2 to sp3
2. sp3 to sp2
3. sp3 to sp3d
4. sp3 to dsp
The shape of covalent molecule AX3 is
1. Triangular
2. T-shape
3. Pyramidal
4. Any of the above three depending upon the number of lone pairs present in A
The geometrical arrangement and shape of are respectively
1. Trigonal bipyramidal geometry, linear shape
2. Hexagonal structure, linear shape
3. Triangular planar geometry, triangular shape
4. Tetrahedral geometry, pyramidal shape
Which has non-zero dipole moment?
1. PCl5
2. PCl3F2
3. PCl3Br2
4. PCl3(CH3)2
Which is incorrect about bond angles?
1. NH3 > NF3
2. NF3 < PF3
3. NH3 > PH3
4. NH3 > H2O
Which molecules/ions are most paramagnetic?
1. B2
2. C2
3.
4.
The most volatile halogen acid is
1. HF
2. HCl
3. HBr
4. HI
The correct statement is
1. When O2 is converted into then bond distance increases
2. When N2 is converted into then bond distance increases
3. When CO is converted into CO+ then bond distance increases
4. All of these
Which has highest bond energy?
1. CO
2. CO+
3. N2
4.
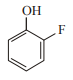
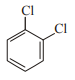
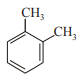
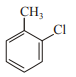
In which case observed dipole moment is greater than theoretical dipole moment?
1. 
2. 
3. 
4. 
Benzene ring contains
1. 12 and 3
2. 6 and 3
3. 9 and 3
4. 8 and 2