Which one of the following matches is correct?
| (a) | Phytophthora | Aseptate Mycelium | Basidiomycetes |
| (b) | Alternaria | Sexual reproduction Absent | Deuteromycetes |
| (c) | Mucor | Reproduction by conjugation | Ascomycetes |
| (d) | Agaricus | Parasitic fungus | Basidiomycetes |
Read the following five statements (I to V) and select the option with all correct statements.
| I: | Mosses and lichens are the first organisms to colonise bare rock. |
| II: | Selaginella is a homosporous pteridophyte. |
| III: | Coralloid roots in Cycas have VAM. |
| IV: | Main plant body in bryophytes is gametophytic, whereas in pteridophytes it is sporophytic. |
| V: | In gymnosperms, male and female gametophytes are present within sporangia located on sporophytes. |
| 1. | I, III and IV | 2. | II, III and IV |
| 3. | I, IV and V | 4. | II, III and V |
In which of the following gametophyte is not independent free-living?
| 1. | Funaria | 2. | Marchantia |
| 3. | Pteris | 4. | Pinus |
Which one of the following statements is wrong?
| 1. | Algin and carrageenan are products of algae |
| 2. | Agar-agar is obtained from Gelidium and Gracilaria |
| 3. | Chlorella and Spirulina are used as space food |
| 4. | Mannitol is stored food in Rhodophyceae |
The guts of a cow and a buffalo possess:
1. Fucus sp
2. Chlorella sp
3. methanogens
4. cyanobacteria
Male gametes are flagellated in:
| 1. | Polysiphonia | 2. | Anabaena |
| 3. | Ectocarpus | 4. | Spirogyra |
Vascular bundles in monocotyledons are considered closed because:
| 1. | a bundle sheath surrounds each bundle |
| 2. | cambium is absent |
| 3. | there are no vessels with perforations |
| 4. | xylem is surrounded all around by phloem |
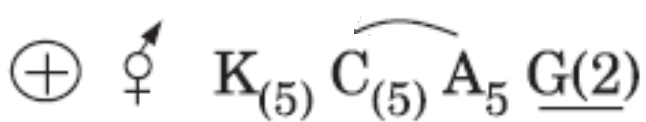 is the floral formula of:
is the floral formula of:
A major characteristic of the monocot root is the presence of:
| 1. | Scattered vascular bundles |
| 2. | Vasculature without cambium |
| 3. | Cambium sandwiched between phloem and xylem along the radius |
| 4. | Open vascular bundles |
Keel is the characteristic feature of a flower of:
Perigynous flowers are found in:
| 1. | guava | 2. | cucumber |
| 3. | China rose | 4. | rose |
Leaves become modified into spines in the:
The structures that are formed by stacking of organised flattened membranous sacks in the chloroplasts are:
The chromosomes in which the centromere is situated close to one end are:
Select the correct matching among the following pairs.
| 1. | Smooth ER-Oxidation of phospholipids |
| 2. | Smooth ER-Synthesis of lipids |
| 3. | Rough ER-Synthesis of glycogen |
| 4. | Rough ER-Oxidation of fatty acids |
Which one of the following is not an inclusion body found in prokaryotes?
| 1. | Phosphate granule | 2. | Cyanophycean granule |
| 3. | Glycogen granule | 4. | Polysome |
Transpiration and root pressure cause water to rise in plants by:
Minerals known to be required in large amounts for plant growth include:
1. phosphorus, potassium, sulphur, calcium
2. calcium, magnesium, manganese, copper
3. potassium, phosphorus, selenium, boron
4. magnesium, sulphur, iron, zinc
What causes a green plant exposed to the light on only one side, to bend toward the source of light as it grows?
| 1. | Green plants need light to perform photosynthesis |
| 2. | Green plants seek light because they are phototropic |
| 3. | Light stimulates plant cells on the lighted side to grow faster |
| 4. | Auxin accumulates on the shaded side, stimulating greater cell elongation there |
In a ring-girdled plant:
The typical growth curve in plants is:
The hilum is a scar on the:
Which one of the following may require pollinators, but is genetically similar to autogamy?
| 1. | Geitonogamy | 2. | Xenogamy |
| 3. | Apogamy | 4. | Cleistogamy |
Which one of the following statements is not true?
| 1. | Pollen grains are rich in nutrients and they are used in the form of tablets and syrups |
| 2. | Pollen grains of some plants cause severe allergies and bronchial afflictions in some people |
| 3. | The flowers pollinated by flies and beetles secrete foul odour to attract them |
| 4. | Honey is made by bees by digesting pollen collected from flowers |
Transmission tissue is a characteristic feature of:
In ginger vegetative propagation occurs through:
Which of the following are the important floral rewards to animal pollinators?
How many pairs of contrasting characters in pea plants were studied by Mendel in his experiments?
| 1. | Five | 2. | Six |
| 3. | Eight | 4. | Seven |
Which is the most common mechanism of genetic variation in the population of a sexually reproducing organism?
A technique of micro-propagation is:
The movement of a gene from one linkage group to another is called:
| 1. | inversion | 2. | duplication |
| 3. | translocation | 4. | crossing over |
Multiple alleles are present:
1. on different chromosomes
2. at different loci on the same chromosome
3. at the same locus of the chromosome
4. on non-sister chromatids
Which body of the Government of India regulates GM research and the safety of introducing GM organisms for public services?
In Bt cotton, the Bt toxin present in plant tissue as pro-toxin is converted into active toxin due to:
The crops engineered for glyphosate are resistant/tolerant to:
DNA is not present in:
Which of the following enhances or induces the fusion of protoplasts?
The UN Conference of Parties on climate change in the year 2011 was held in:
The vertical distribution of different species occupying different levels in a biotic community is known as:
In which of the following do both pairs have the correct combination?
| 1. | In situ conservation/National park Ex-situ conservation/Botanical garden |
| 2. | In situ conservation/Cryopreservation Ex-situ conservation/Wildlife sanctuary |
| 3. | In situ conservation/Seed bank Ex-situ conservation/ National park |
| 4. | In situ conservation/Tissue culture Ex-situ conservation/Sacred groves |
Secondary succession takes place on/in:
The mass of living material at a tropic level at a particular time is called:
In an ecosystem, the rate of production of organic matter during photosynthesis is termed as:
1. net primary productivity
2. gross primary productivity
3. secondary productivity
4. net productivity
Which of the following characteristics is mainly responsible for the diversification of insects on land:
| 1. | Segmentation | 2. | Bilateral symmetry |
| 3. | Exoskeleton | 4. | Eyes |
Which of the following endoparasites of humans show viviparity?
1. Ancylostoma duodenale
2. Enterobius vermicularis
3. Trichinella spiralis
4. Ascaris lumbricoides
Which of the following represents the correct combination without any exception?
| Characteristic | Class | |
| (a) | Mammary gland; hair on body; pinnae; two pairs of limbs | Mammalia |
| (b) | Mouth ventral; gills without operculum; skin with placoid scales; persistent notochord | Chondrichthyes |
| (c) | Sucking and circular mouth; jaws absent; integument without scales; paired appendages | Cyclostomata |
| (d) | Body covered with feathers; skin moist and glandular; lungs with air sacs; forelimbs from wings | Aves |
| 1. | (a) | 2. | (b) |
| 3. | (c) | 4. | (d) |
Erythropoiesis starts in:
| 1. | kidney | 2. | liver |
| 3. | spleen | 4. | red bone marrow |
The terga,sterna and pleura of the cockroach body are joined by:
1. cementing glue
2. muscular tissue
3. arthrodial membrane
4. cartilage
The nuclear envelope is derivative of:
| 1. | smooth endoplasmic reticulum |
| 2. | membrane of Golgi complex |
| 3. | microtubules |
| 4. | rough endoplasmic reticulum |
Cytochromes are found in:
| 1. | matrix of mitochondria |
| 2. | outer wall of mitochondria |
| 3. | cristae of mitochondria |
| 4. | lysosomes |
Which one of the following statements is incorrect?
| 1. | A competitive inhibitor reacts reversibly with the enzyme to form an enzyme inhibitor. |
| 2. | In competitive inhibition, the inhibitor molecule is not chemically changed by the enzyme. |
| 3. | The competitive inhibitor does not affect the rate of breakdown of the enzyme-substrate complex. |
| 4. | The presence of the competitive inhibitor decreases the km of the enzyme for the substrate. |
Match the following Column-I with Column-II.
| Column-I | Column-II | ||
| A. | Synapsis aligns homologous chromosomes |
(i) | Anaphase II |
| B. | Synthesis of RNA and protein | (ii) | Zygotene |
| C. | Action of enzyme recombinase | (iii) | G2 - phase |
| D. | Centromeres do not separate, but chromosomes move towards opposite poles | (iv) | Anaphase I |
| (v) | Pachytene |
| 1. | A-(ii), B-(i), C-(iii), D-(iv) | 2. | A-(ii), B-(iii), C-(v), D-(iv) |
| 3. | A-(i), B-(ii), C-(v), D-(iv) | 4. | A-(ii), B-(iii), C-(iv), D-(v) |
A somatic cell that has just completed the S-phase of its cell cycle, as compared to a gamete of the same species has:
| 1. | twice the number of chromosomes and twice the amount of DNA |
| 2. | the same number of chromosomes but twice the amount of DNA |
| 3. | twice the number of chromosomes and four times the amount of DNA |
| 4. | four times the number of chromosomes and twice the amount of DNA |
Which of the following statement is not correct?
| 1. | Brunner's glands are present in the submucosa of stomach and secrete pepsinogen |
| 2. | Goblet cells are present in the mucosa of the intestine and secrete mucus |
| 3. | Oxyntic cells are present in the mucosa of the stomach and secrete HCI |
| 4. | Acini are present in the pancreas and secrete carboxypeptidase |
The gastric juice of infants contains:
When you hold your breath, which of the following gas changes in blood would first lead to the urge to breathe?
1. Falling O2 concentration
2. Rising CO2 concentration
3. Falling CO2 concentration
4. Rising CO2 and falling O2 concentration
Blood pressure in the mammalian aorta is maximum during:
Which one of the following is correct?
1. Serum = Blood + Fibrinogen
2. Lymph = Plasma + RBC + WBC
3. Blood = Plasma + RBC + WBC + Platelets
4. Plasma = Blood – Lymphocytes
Removal of the proximal convoluted tubule from the nephron will result in:
Sliding filament theory can be best explained as:
| 1. | when myofilaments slide past each other, actin filaments shorten while myosin filament does not shorten |
| 2. | actin and myosin filaments shorten and slide past each other |
| 3. | actin and myosin filaments do not shorten but rather slide pass each other |
| 4. | when myofilaments slide past each other, myosin filaments shorten while actin filaments do not shorten |
Glenoid cavity articulates:
1. clavicle with acromion
2. scapula with acromion
3. clavicle with scapula
4. humerus with scapula
Which of the following regions of the brain is incorrectly paired with its function?
| 1. | Medulla oblongata-Homeostatic control |
| 2. | Cerebellum-Language comprehension |
| 3. | Corpus callosum-Communication between the left and right cerebral cortices |
| 4. | Cerebrum-Calculation and contemplation |
A gymnast is able to balance his body upside down even in total darkness because of:
1. cochlea
2. vestibular apparatus
3. tectorial membrane
4. the organ of Corti
A chemical signal that has both endocrine and neural roles is:
Which of the following does not favour the formation of large quantities of dilute urine?
Capacitation refers to changes in the:
Which of the following viruses is not transferred through the semen of an infected male?
| 1. | Hepatitis-B virus | 2. | Human immunodeficiency virus |
| 3. | Chikungunya virus | 4. | Ebola virus |
Which of the following cells during gametogenesis is normally diploid?
Hysterectomy is the surgical removal of:
| 1. | uterus | 2. | prostate gland |
| 3. | vas deference | 4. | mammary glands |
Which of the following is not a sexually transmitted disease?
1. Syphilis
2. Acquired Immuno Deficiency Syndrome (AIDS)
3. Trichomoniasis
4. Encephalitis
An abnormal human baby with 'XXX' sex chromosomes was born due to:
1. formation of abnormal ova in the mother
2. fusion of two ova and one sperm
3. fusion of two sperms and one ovum
4. formation of abnormal sperms in the father
Alleles are:
1. different phenotype
2. true breeding homozygotes
3. different molecular forms of a gene
4. heterozygotes
A man with blood group 'A' marries a woman with blood group 'B'. What are all the possible blood groups of their offspring?
Gene regulation governing lactose operon of E. coli that involves the lac I gene products is:
| 1. | positive and inducible because it can be induced by lactose. |
| 2. | negative and inducible because repress or protein prevents transcription. |
| 3. | negative and repressible because repress or protein prevents transcription. |
| 4. | feedback inhibition because excess of β galactosidase can switch off transcription. |
In sea urchin DNA, which is double-stranded 17% of the bases were shown to be cytosine. The percentages of the other three bases expected to be present in this DNA are:
Which of the following had the smallest brain capacity?
1. Homo erectus
2. Homo sapiens
3. Homo neanderthalensis
4. Homo habilis
Match each disease with its correct type of vaccine:
| Column-I | Column-II | ||
| A. | Tuberculosis | 1. | Harmless virus |
| B. | Whooping cough | 2. | Inactivated toxin |
| C. | Diptheria | 3. | Killed bacteria |
| D. | Polio | 4. | Harmless bacteria |
| 1. | A-2 B-1 C-3 D-4 | 2. | A-3 B-2 C-4 D-1 |
| 3. | A-4 B-3 C-2 D-1 | 4. | A-1 B-2 C-4 D-3 |
The active form of Entamoeba histolytica feeds upon:
A high value of BOD (Biochemical Oxygen Demand) indicates that:
| 1. | water is pure |
| 2. | water is highly polluted |
| 3. | water is less polluted |
| 4. | consumption of organic matter in the water is higher by the microbes |
Most animals are tree dwellers in a:
The following graph depicts changes in two populations (A and B) of herbivores in a grassy field. A possible reason for these changes is that:
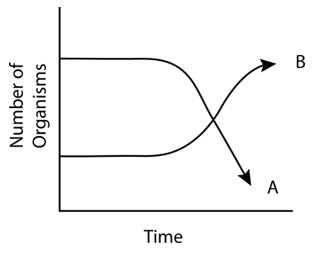
| 1. | both plant populations in this habitat decreased |
| 2. | population-B competed more successfully for food than population-A |
| 3. | population-A produced more offspring than population-B |
| 4. | population-A consumed the members of population-B |
Cryopreservation of gametes of threatened species in viable and fertile conditions can be referred to as:
| 1. | Advanced ex-situ conservation of biodiversity |
| 2. | In situ conservation by sacred groves |
| 3. | In situ cryo-conservation of biodiversity |
| 4. | In situ conservation of biodiversity |
Rachel Carson's famous book 'Silent Spring' is related to:
Which of the following is not one of the prime health risks associated with greater UV radiation through the atmosphere due to the depletion of the stratospheric zone?
Which of the following species contains an equal number of and bonds?
1.
2.
3.
4. CH2(CN)2
The species Ar, K+ and Ca2+ contain the same number of electrons. In which order do their radii increase?
1. Ar < K+ < Ca2+
2. Ca2+ < Ar < K+
3. Ca2+ < K+ < Ar
4. K+ < Ar < Ca2+
Which of the following biologically important ions is also a constituent of the sodium pump?
1. Ca2+
2. Mg2+
3. K+
4. Fe2+
"Metals are usually not found as nitrates in their ores". Out of the following two (I and II) reasons which is/are true for the above observation?
| I: | Metal nitrates are highly unstable. |
| II: | Metal nitrates are highly soluble in water. |
Solubility of alkaline earth metal sulphates in water decreases in the sequence:
1. Mg > Ca > Sr >Ba
2. Ca > Sr > Ba > Mg
3. Sr > Ca > Mg > Ba
4. Ba > Mg > Sr > Ca
A pair among the following have nearly the same atomic radii:
(Numbers in the parenthesis are atomic numbers).
| 1. | Ti (22) and Zr (40) | 2. | Zr (40) and Nb (41) |
| 3. | Zr (40) and Hf (72) | 4. | Zr (40) and Ta (73) |
Which of the following processes does not involve the oxidation of iron?
| 1. | Rusting of iron sheets |
| 2. | Decolourisation of blue CuSO4 solution by iron |
| 3. | Formation of Fe(CO)5 from Fe |
| 4. | Liberation of H2 from steam by iron at high temperature |
| 1. | \(\mathrm{CO}_3^{2-}, \mathrm{SO}_3^{2-} \) | 2. | \(\mathrm{ClO}_3^{-}, \mathrm{CO}_3^{2-} \) |
| 3. | \(\mathrm{SO}_3^{2-}, \mathrm{NO}_3^{-} \) | 4. | \(\mathrm{ClO}_3^{-}, \mathrm{SO}_3^{2-}\) |
Treatment of cyclopentanone with methyl lithium generates:
1. Cyclopentanonyl cation
2. Cyclopentanonyl radical
3. Cyclopentanonyl biradical
4. Cyclopentanonyl anion
Choose the compound which has a maximum bond angle at nitrogen among the following:
| 1. | 2. | ||
| 3. | 4. |
An ion, among the following, that has a magnetic moment of 2.84 BM is:
(At. no. Ni = 28, Ti = 22, Cr = 24, Co = 27)
1. Ni2+
2. Ti3+
3. Cr2+
4. Co2+
Cobalt(III) chloride forms several octahedral complexes with ammonia.
A compound among the following that does not give a test for chloride ions with silver nitrate at 25 °C is:
| 1. | CoCl3. 3NH3 | 2. | CoCl3. 4NH3 |
| 3. | CoCl3. 5NH3 | 4. | CoCl3. 6NH3 |
The correct statement, among the following, is:
| 1. | [Co(CN)6]3- has no unpaired electrons and will be in a low-spin configuration. |
| 2. | [Co(CN)6]3- has four unpaired electrons and will be in a low-spin configuration. |
| 3. | [Co(CN)6]3- has four unpaired electrons and will be In a high-spin configuration. |
| 4. | [Co(CN)6]3- has no unpaired electrons and will be in a high-spin configuration. |
| 1. | In K vs T |
| 2. | In \(\frac K T\) vs T |
| 3. | In K vs \(\frac 1 T\) |
| 4. | In \(\frac T K\) vs \(\frac 1 T\) |
A mixture of gases contains H2 and O2 gases in the ratio of 1 : 4 (w/w). The molar ratio of the two gases in the mixture will be:
| 1. | 1:4 | 2. | 4:1 |
| 3. | 16:1 | 4. | 2:1 |
A given metal crystallizes out with a cubic structure having an edge length of 361 pm. If there are four metal atoms in one unit cell, the radius of one atom is:
1. 40 pm
2. 127 pm
3. 80 pm
4. 108 pm
When the initial concentration of a reactant is doubled in a reaction, its half-life period is not affected. The order of the reaction will be:
1. 0
2. 1
3. 1.5
4. 2
The value of the equilibrium constant for a particular reaction is 1.6 × 1012. When the system is in equilibrium, it will include:
1. All reactants
2. Mostly reactants
3. Mostly products
4. Similar amounts of reactants and products
A device that converts the energy of combustion of fuels, like hydrogen and methane, directly into electrical energy is known as:
| 1. | Fuel cell. | 2. | Electrolytic cell. |
| 3. | Dynamo. | 4. | Ni-Cd cell. |
The boiling point of 0.2 mol kg–1 solution of X in water is greater than the equimolal solution of Y in water. The correct statement in this case is:
| 1. | X is undergoing dissociation in water. |
| 2. | Molecular mass of X is greater than the molecular mass of Y. |
| 3. | Molecular mass of X is less than the molecular mass of Y. |
| 4. | Y is undergoing dissociation in water while X undergoes no change. |
The electrolyte having the same value of Van't Hoff factor (i) as that of Al2(SO4)3 (if all are 100% ionized) is:
| 1. | K2SO4 | 2. | K3[Fe(CN)6] |
| 3. | Al(NO3)3 | 4. | K4[Fe(CN)6] |
The number of d -electrons in Fe2+ (atomic number Z = 26) is different from the number of:
1. s-electrons in Mg (Z = 12)
2. p-electrons in Cl (Z = 17)
3. d-electrons in Fe (Z = 26)
4. p-electrons in Ne (Z = 10)
The correct order of bond order in the following species is:
| 1. | \(\mathrm{O}^{2+}_2>\mathrm{O}^+_2>\mathrm{O}^-_2\) | 2. | \(\mathrm{O}^{2+}_2<\mathrm{O}^-_2<\mathrm{O}^+_2\) |
| 3. | \(\mathrm{O}^{+}_2>\mathrm{O}^-_2<\mathrm{O}^{2+}_2\) | 4. | \(\mathrm{O}^{-}_2<\mathrm{O}^+_2>\mathrm{O}^{2+}_2\) |
The angular momentum of electrons in d orbital is equal to:
1.
2.
3.
4. 0
The Ksp of Ag2CrO4, AgCl, AgBr, and Agl are respectively, 1.1 × 10–12, 1.8 × 10–10, 5.0 × 10–13, 8.3 × 10–17. Which one of the following salts will precipitate last if solution is added to the solution containing equal moles of NaCl, NaBr, Nal, and Na2CrO4?
1. Agl
2. AgCl
3. AgBr
4. Ag2CrO4
Which property of the colloidal solution is independent of charge on the colloidal particles?
The correct statement for a reversible process in a state of equilibrium is:
1. G = – 2.30RT log K
2. G = 2.30RT log K
3. Go = – 2.30RT log K
4. Go = 2.30RT log K
Bithional is generally added to the soaps as an additive to function as a/an:
What results from the electrolytic reduction of nitrobenzene in a highly acidic medium?
1. p-Aminophenol
2. Azoxybenzene
3. Azobenzene
4. Aniline
In Duma's method for estimation of nitrogen, 0.25 g of an organic compound gave 40 mL of nitrogen collected at 300 K temperature and 725 mm pressure. If the aqueous tension at 300 K is 25 mm, the percentage of nitrogen in the compound is:
| 1. | 17.36 | 2. | 18.20 |
| 3. | 16.76 | 4. | 15.76 |
In which of the following compounds, the C—Cl bond ionization shall give the most stable carbonium ion?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The reaction,
is called:
1. Williamson synthesis
2. Williamson's continuous etherification process
3. Etard reaction
4. Gatterman-Koch reaction
The reaction of C6H5CH =CHCH3 with HBr produces:
| 1. | \(C_{6} H_{5} \underset{Br}{\underset{\left|\right.}{CH}} CH_{2} CH_{3} \) |
| 2. | \(C_{6} H_{5} CH_{2} \underset{Br}{\underset{\left|\right.}{CH}} CH_{3} \) |
| 3. | \(C_{6} H_{5} CH_{2} CH_{2} CH_{2} Br \) |
| 4. |  |
When subjected to ozonolysis, which compound results in the formation of the given molecule?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Hyperconjugation occurs among the following compounds:
| 1. | I only | 2. | II only |
| 3. | III only | 4. | I and III |
Which of the following is the most correct electron displacement for a nucleophilic reaction to take place?
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
The enolic form of ethyl acetoacetate is given below:
The number of σ and π bonds in the enolic form are respectively:
1. 18 sigma bonds and 2 pi-bonds
2. 16 sigma bonds and 1 pi-bond
3. 9 sigma bonds and 2 pi-bonds
4. 9 sigma bonds and 1 pi-bond
Compounds that can exhibit tautomerism are:
| 1. | l and ll | 2. | l and lll |
| 3. | ll and lll | 4. | l, ll and lll |
Given compounds are as follows:
| (I) |  |
(II) |  |
| (III) |  |
The enthalpy of hydrogenation of these compounds will be in the order as-
| 1. | I > II > III | 2. | III > II > I |
| 3. | II > III > I | 4. | II > I > III |
A biodegradable polymer that can be prepared from glycine and aminocaproic acid is:
1. Nylon 2-nylon 6
2. PHBV
3. Buna-N
4. Nylon-6,6
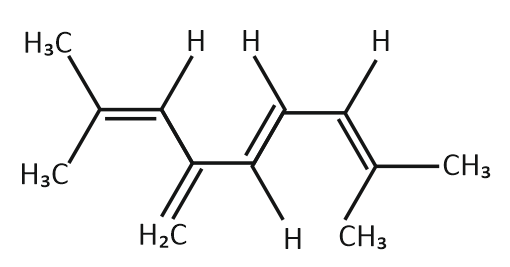
| 1. | Four (4) | 2. | Eight (8) |
| 3. | Twelve (12) | 4. | Sixteen (16) |
An organic compound X having molecular formula C5H10O yields phenyl hydrazone and gives a negative response to the iodoform test and Tollen's test. It produces n-pentane on reduction. X could be:
A ship \(A\) is moving westward with a speed of \(10~\text{kmph}\) and a ship \(B,\) \(100 ~\text{km}\) south of \(A,\) is moving northward with a speed of \(10~\text{kmph}.\) The time after which the distance between them becomes the shortest is:
| 1. | \(0~\text{h}\) | 2. | \(5~\text{h}\) |
| 3. | \(5\sqrt{2}~\text{h}\) | 4. | \(10\sqrt{2}~\text{h}\) |
| 1. | \(- 2 nβ^{2} x^{- 2 n - 1}\) | 2. | \(- 2 nβ^{2} x^{- 4 n - 1}\) |
| 3. | \(- 2 \beta^{2} x^{- 2 n + 1}\) | 4. | \(- 2 nβ^{2} x^{- 4 n + 1}\) |
Three blocks \(\mathrm{A}\), \(\mathrm{B}\), and \(\mathrm{C}\) of masses \(4~\text{kg}\), \(2~\text{kg}\), and \(1~\text{kg}\) respectively, are in contact on a frictionless surface, as shown. If a force of \(14~\text{N}\) is applied to the \(4~\text{kg}\) block, then the contact force between \(\mathrm{A}\) and \(\mathrm{B}\) is:
1. \(2~\text{N}\)
2. \(6~\text{N}\)
3. \(8~\text{N}\)
4. \(18~\text{N}\)
A block \(\mathrm{A}\) of mass \(m_1\) rests on a horizontal table. A light string connected to it passes over a frictionless pulley at the edge of the table and from its other end, another block \(\mathrm{B}\) of mass \(m_2\) is suspended. The coefficient of kinetic friction between block \(\mathrm{A}\) and the table is \(\mu_k\). When block \(\mathrm{A}\) is sliding on the table, the tension in the string is:
| 1. | \( \dfrac{\left({m}_2+\mu_{{k}}{m}_1\right) {g}}{\left({m}_1+{m}_2\right)}\) | 2. | \( \dfrac{\left({m}_2-\mu_{{k}} {m}_1\right) {g}}{\left({m}_1+{m}_2\right)}\) |
| 3. | \(\dfrac{{m}_1 {~m}_2\left(1-\mu_{{k}}\right) {g}}{\left({m}_1+{m}_2\right)}\) | 4. | \( \dfrac{{m}_1 {~m}_2\left(1+\mu_{{k}}\right)}{{m}_1+{m}_2} {g}\) |
Two similar springs \(P\) and \(Q\) have spring constants \(k_P\) and \(k_Q\), such that \(k_P>k_Q\). They are stretched, first by the same amount (case a), then by the same force (case b). The work done by the springs \(W_P\) and \(W_Q\) are related as, in case (a) and case (b), respectively:
| 1. | \(W_P=W_Q;~W_P>W_Q\) |
| 2. | \(W_P=W_Q;~W_P=W_Q\) |
| 3. | \(W_P>W_Q;~W_P<W_Q\) |
| 4. | \(W_P<W_Q;~W_P<W_Q\) |
| 1. | \(475\) J | 2. | \(450\) J |
| 3. | \(275\) J | 4. | \(250\) J |
| 1. | \( \sqrt{\frac{m k}{2}} t^{-1 / 2} \) | 2. | \( \sqrt{m k} t^{-1 / 2} \) |
| 3. | \( \sqrt{2 m k} t^{-1 / 2} \) | 4. | \( \frac{1}{2} \sqrt{m k} t^{-1 / 2}\) |
Two particles of masses \(m_1\) and \(m_2\) move with initial velocities \(u_1\) and \(u_2\) respectively. On collision, one of the particles gets excited to a higher level, after absorbing energy \(E\). If the final velocities of particles are \(v_1\) and \(v_2\), then we must have:
| 1. | \(m_1^2u_1+m_2^2u_2-E = m_1^2v_1+m_2^2v_2\) |
| 2. | \(\frac{1}{2}m_1u_1^2+\frac{1}{2}m_2u_2^2= \frac{1}{2}m_1v_1^2+\frac{1}{2}m_2v_2^2\) |
| 3. | \(\frac{1}{2}m_1u_1^2+\frac{1}{2}m_2u_2^2-E= \frac{1}{2}m_1v_1^2+\frac{1}{2}m_2v_2^2\) |
| 4. | \(\frac{1}{2}m_1^2u_1^2+\frac{1}{2}m_2^2u_2^2+E = \frac{1}{2}m_1^2v_1^2+\frac{1}{2}m_2^2v_2^2\) |
| 1. | \(wx \over d\) | 2. | \(wd \over x\) |
| 3. | \(w(d-x) \over x\) | 4. | \(w(d-x) \over d\) |
A mass \(m\) moves in a circle on a smooth horizontal plane with velocity \(v_0\) at a radius \(R_0.\) The mass is attached to a string that passes through a smooth hole in the plane, as shown in the figure.
The tension in the string is increased gradually and finally, \(m\) moves in a circle of radius \(\frac{R_0}{2}.\) The final value of the kinetic energy is:
| 1. | \( m v_0^2 \) | 2. | \( \dfrac{1}{4} m v_0^2 \) |
| 3. | \( 2 m v_0^2 \) | 4. | \( \dfrac{1}{2} m v_0^2\) |
Three identical spherical shells, each of mass \(m\) and radius \(r\) are placed as shown in the figure. Consider an axis \(XX',\) which is touching two shells and passing through the diameter of the third shell. The moment of inertia of the system consisting of these three spherical shells about the \(XX'\) axis is:
| 1. | \(\dfrac{11}{5}mr^2\) | 2. | \(3mr^2\) |
| 3. | \(\dfrac{16}{5}mr^2\) | 4. | \(4mr^2\) |
Kepler's third law states that the square of the period of revolution (\(T\)) of a planet around the sun, is proportional to the third power of average distance \(r\) between the sun and planet i.e. \(T^2 = Kr^3\), here \(K\) is constant. If the masses of the sun and planet are \(M\) and \(m\) respectively, then as per Newton's law of gravitation, the force of attraction between them is \(F = \frac{GMm}{r^2},\) here \(G\) is the gravitational constant. The relation between \(G\) and \(K\) is described as:
1. \(GK = 4\pi^2\)
2. \(GMK = 4\pi^2\)
3. \(K =G\)
4. \(K = \frac{1}{G}\)
Two spherical bodies of masses \(M\) and \(5M\) and radii \(R\) and \(2R\) are released in free space with initial separation between their centres equal to \(12R.\) If they attract each other due to gravitational force only, then the distance covered by the smaller body before the collision is:
| 1. | \(2.5R\) | 2. | \(4.5R\) |
| 3. | \(7.5R\) | 4. | \(1.5R\) |
On observing light from three different stars \(P,\) \(Q,\) and \(R,\) it was found that the intensity of the violet colour is maximum in the spectrum of \(P,\) the intensity of the green colour is maximum in the spectrum of \(R\) and the intensity of the red colour is maximum in the spectrum of \(Q.\) If \(T_P,\) \(T_Q,\) and \(T_R\) are the respective absolute temperatures of \(P,\) \(Q,\) and \(R,\) then it can be concluded from the above observations that:
1. \(T_P>T_Q>T_R\)
2. \(T_P>T_R>T_Q\)
3. \(T_P<T_R<T_Q\)
4. \(T_P<T_Q<T_R\)
The approximate depth of an ocean is \(2700~\text{m}\). The compressibility of water is \(45.4\times10^{-11}~\text{Pa}^{-1}\) and the density of water is \(10^{3}~\text{kg/m}^3\). What fractional compression of water will be obtained at the bottom of the ocean?
| 1. | \(0.8\times 10^{-2}\) | 2. | \(1.0\times 10^{-2}\) |
| 3. | \(1.2\times 10^{-2}\) | 4. | \(1.4\times 10^{-2}\) |
The two ends of a metal rod are maintained at temperatures \(100~^\circ\text{C}\) and \(110~^\circ\text{C}.\) The rate of heat flow in the rod is found to be \(4.0\) J/s. If the ends are maintained at temperatures \(200~^\circ \text{C}\) and \(210 ~^\circ \text{C},\) the rate of heat flow will be:
| 1. | \(44.0\) J/s | 2. | \(16.8\) J/s |
| 3. | \(8.0\) J/s | 4. | \(4.0\) J/s |
| 1. | \(4 \times 10^5~\text N,\) downwards | 2. | \(4 \times 10^5~\text N,\) upwards |
| 3. | \(2.4 \times 10^5~\text N,\) upwards | 4. | \(2.4 \times 10^5~\text N,\) downwards |
The figure below shows two paths that may be taken by gas to go from state \(A\) to state \(C.\)

In process \(AB,\) \(400~\text{J}\) of heat is added to the system, and in process \(BC,\) \(100~\text{J}\) of heat is added to the system. The heat absorbed by the system in the process \(AC\) will be:
1. \(380~\text{J}\)
2. \(500~\text{J}\)
3. \(460~\text{J}\)
4. \(300~\text{J}\)
A Carnot engine, having an efficiency of = as a heat engine, is used as a refrigerator. If the work done on the system is \(10\) J, the amount of energy absorbed from the reservoir at a lower temperature is:
1. \(100\) J
2. \(99\) J
3. \(90\) J
4. \(1\) J
One mole of an ideal diatomic gas undergoes a transition from \(A\) to \(B\) along a path \(AB\) as shown in the figure.
The change in internal energy of the gas during the transition is:
| 1. | \(20~\text{kJ}\) | 2. | \(-20~\text{kJ}\) |
| 3. | \(20~\text{J}\) | 4. | \(-12~\text{kJ}\) |
| 1. | \(\left(1+\frac{1}{n}\right )\) | 2. | \(\left(1+\frac{n}{3}\right)\) |
| 3. | \(\left(1+\frac{2}{n}\right)\) | 4. | \(\left(1+\frac{n}{2}\right)\) |
When two displacements are represented by \(y_1 = a \text{sin}(\omega t)\) and \(y_2 = b\text{cos}(\omega t)\) are superimposed, then the motion is:
| 1. | not simple harmonic. |
| 2. | simple harmonic with amplitude \(\dfrac{a}{b}\). |
| 3. | simple harmonic with amplitude \(\sqrt{a^2+b^{2}}.\) |
| 4. | simple harmonic with amplitude \(\dfrac{a+b}{2}\). |
A particle is executing SHM along a straight line. Its velocities at distances \(x_1\) and \(x_2\) from the mean position are \(v_1\) and \(v_2\), respectively. Its time period is:
| 1. | \(2 \pi \sqrt{\dfrac{x_{1}^{2}+x_{2}^{2}}{v_{1}^{2}+v_{2}^{2}}}~\) | 2. | \(2 \pi \sqrt{\dfrac{{x}_{2}^{2}-{x}_{1}^{2}}{{v}_{1}^{2}-{v}_{2}^{2}}}\) |
| 3. | \(2 \pi \sqrt{\dfrac{v_{1}^{2}+v_{2}^{2}}{x_{1}^{2}+x_{2}^{2}}}\) | 4. | \(2 \pi \sqrt{\dfrac{v_{1}^{2}-v_{2}^{2}}{x_{1}^{2}-x_{2}^{2}}}\) |
The fundamental frequency of a closed organ pipe of a length \(20\) cm is equal to the second overtone of an organ pipe open at both ends. The length of the organ pipe open at both ends will be:
| 1. | \(80\) cm | 2. | \(100\) cm |
| 3. | \(120\) cm | 4. | \(140\) cm |
A parallel plate air capacitor of capacitance \(C\) is connected to a cell of emf \(V\) and then disconnected from it. A dielectric slab of dielectric constant \(K,\) which can just fill the air gap of the capacitor is now inserted in it. Which of the following is incorrect?
| 1. | The potential difference between the plates decreases \(K\) times. |
| 2. | The energy stored in the capacitor decreases \(K\) times. |
| 3. | The change in energy stored is \(\frac{1}{2}CV^{2}\left ( \dfrac{1}{K} -1\right ) \) |
| 4. | The charge on the capacitor is not conserved. |
The electric field in a certain region is acting radially outward and is given by \(E=Aa.\) A charge contained in a sphere of radius \(a\) centered at the origin of the field will be given by:
| 1. | \(4 \pi \varepsilon_{{o}} {A}{a}^2\) | 2. | \(\varepsilon_{{o}} {A} {a}^2\) |
| 3. | \(4 \pi \varepsilon_{{o}} {A} {a}^3\) | 4. | \(\varepsilon_{{o}} {A}{a}^3\) |
A potentiometer wire has a length of \(4~\text{m}\) and resistance \(8~\Omega.\) The resistance that must be connected in series with the wire and an energy source of emf \(2~\text{V}\), so as to get a potential gradient of \(1~\text{mV}\) per cm on the wire is:
1. \(32~\Omega\)
2. \(40~\Omega\)
3. \(44~\Omega\)
4. \(48~\Omega\)
\({A, B}~\text{and}~{C}\) are voltmeters of resistance \(R,\) \(1.5R\) and \(3R\) respectively as shown in the figure above. When some potential difference is applied between \({X}\) and \({Y},\) the voltmeter readings are \({V}_{A},\) \({V}_{B}\) and \({V}_{C}\) respectively. Then:

| 1. | \({V}_{A} ={V}_{B}={V}_{C}\) | 2. | \({V}_{A} \neq{V}_{B}={V}_{C}\) |
| 3. | \({V}_{A} ={V}_{B}\neq{V}_{C}\) | 4. | \({V}_{A} \ne{V}_{B}\ne{V}_{C}\) |
| 1. | current density | 2. | current |
| 3. | drift velocity | 4. | electric field |
A wire carrying current \(I\) has the shape as shown in the adjoining figure. Linear parts of the wire are very long and parallel to \(X\)-axis while the semicircular portion of radius \(R\) is lying in the \(Y\text-Z\) plane. The magnetic field at point \(O\) is:

An electron moving in a circular orbit of radius \(r\) makes \(n\) rotations per second. The magnetic field produced at the centre has a magnitude:
| 1. | \(\dfrac{\mu_0ne}{2\pi r}\) | 2. | zero |
| 3. | \(\dfrac{n^2e}{r}\) | 4. | \(\dfrac{\mu_0ne}{2r}\) |
A conducting square frame of side \(a\) and a long straight wire carrying current \(I\) are located in the same plane as shown in the figure. The frame moves to the right with a constant velocity \(v.\) The emf induced in the frame will be proportional to:
| 1. | \( \dfrac{1}{x^2} \) | 2. | \( \dfrac{1}{(2 x-a)^2} \) |
| 3. | \( \dfrac{1}{(2 x+a)^2} \) | 4. | \(\dfrac{1}{(2 x-a)(2 x+a)}\) |
A resistance \(R\) draws power \(P\) when connected to an AC source. If an inductance is now placed in series with the resistance, such that the impedance of the circuit becomes \(Z\), the power drawn will be:
| 1. | \(P\Big({\large\frac{R}{Z}}\Big)^2\) | 2. | \(P\sqrt{\large\frac{R}{Z}}\) |
| 3. | \(P\Big({\large\frac{R}{Z}}\Big)\) | 4. | \(P\) |
Radiation of energy \(E\) falls normally on a perfectly reflecting surface. The momentum transferred to the surface is:
(\(c\) = velocity of light)
| 1. | \(E \over c\) | 2. | \(2E \over c\) |
| 3. | \(2E \over c^2\) | 4. | \(E \over c^2\) |
Two identical thin plano-convex glass lenses (refractive index = \(1.5\)) each having radius of curvature of \(20\) cm are placed with their convex surfaces in contact at the centre. The intervening space is filled with oil of a refractive index of \(1.7\). The focal length of the combination is:
| 1. | \(-20\) cm | 2. | \(-25\) cm |
| 3. | \(-50\) cm | 4. | \(50\) cm |
For a parallel beam of monochromatic light of wavelength \(\lambda\), diffraction is produced by a single slit whose width \(a\) is much greater than the wavelength of the light. If \(D\) is the distance of the screen from the slit, the width of the central maxima will be:
| 1. | \(\dfrac{2D\lambda}{a}\) | 2. | \(\dfrac{D\lambda}{a}\) |
| 3. | \(\dfrac{Da}{\lambda}\) | 4. | \(\dfrac{2Da}{\lambda}\) |
| 1. | \(180^\circ-3A\) | 2. | \(180^\circ-2A\) |
| 3. | \(90^\circ-A\) | 4. | \(180^\circ+2A\) |
| 1. | \(6\lambda\) | 2. | \(4\lambda\) |
| 3. | \(\dfrac{\lambda}{4}\) | 4. | \(\dfrac{\lambda}{6}\) |
Which of the following figures represent the variation of the particle momentum and the associated de-Broglie wavelength?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Consider \(3^{\text{rd}}\) orbit of \(He^{+}\) (Helium). Using a non-relativistic approach, the speed of the electron in this orbit will be: (given \(Z=2\) and \(h\) (Planck's constant)\(= 6.6\times10^{-34}~\text{J-s}\))
1. \(2.92\times 10^{6}~\text{m/s}\)
2. \(1.46\times 10^{6}~\text{m/s}\)
3. \(0.73\times 10^{6}~\text{m/s}\)
4. \(3.0\times 10^{8}~\text{m/s}\)
If the radius of \(_{13}^{27}\mathrm{Al}\) nucleus is taken to be \({R}_{\mathrm{Al}},\) then the radius of \(_{53}^{125}\mathrm{Te}\) nucleus is near:
| 1. | \(\left(\frac{53}{13}\right) ^{\frac{1}{3}}~{R_{Al}}\) | 2. | \(\frac{5}{3}~{R_{Al}}\) |
| 3. | \(\frac{3}{5}~{R_{Al}}\) | 4. | \(\left(\frac{13}{53}\right)~{R_{Al}}\) |
If in a \(\mathrm{p\text{-}n}\) junction, a square input signal of \(10~\text{V}\) is applied as shown,
then the output across \(R_L\) will be:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Which logic gate is represented by the following combination of logic gates?
