Which of the following compounds with molecular formula, C5H10 yields acetone on ozonolysis?
1. 2-methyl-2-butene
2. 3-methyl-1-butene
3. Cyclopentane
4. 2-methyl-1-butene
A compound is treated with to give sodium salt. Identify the compound.
1.
2.
3.
4.
What product(s) will be obtained from the ozonolysis of the compound shown below?

| 1. |  |
2. |  |
| 3. |  |
4. | None of the above |
A compound that gives an optically inactive compound on treatment with H2(2 moles)/ Pt is-
1. 3-Methyl-1-pentyne
2. 4-Methyl-1-hexyne
3. 3-Methyl-1-heptyne
4. None of the above
The reaction of toluene with chlorine in the presence of light gives:
| 1. |  |
2. |  |
| 3. |  |
4. |  |

The Product (B) in the above-mentioned reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The product B in the above-mentioned reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
In the following reactions:-
the major products (A) and (C) are respectively
Benzene reacts with CH3Cl in the presence of anhydrous AlCl3 to form.
1. toluene
2. chlorobenzene
3. benzylchloride
4. xylene
Which of the following compounds will react with ammoniacal silver nitrate AgNO3?
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
What is the correct order of acidity among the following compounds?
| 1. | \(\small\mathrm{CH \equiv CH > CH_3 - C \equiv CH }~ \mathrm{ > CH_2 = CH_2 > CH_3 - CH_3}\) |
| 2. | \(\small\mathrm{CH \equiv CH > CH_2 = CH_2 }~ \mathrm{ > CH_3 - C \equiv CH > CH_3 - CH_3}\) |
| 3. | \(\small\mathrm{CH_3 - CH_3 > CH_2 = CH_2 }~ \mathrm{ > CH_3 - C \equiv CH > CH \equiv CH }\) |
| 4. | \(\small\mathrm{ CH_2 = CH_2 > CH_3 - CH_3 } ~\mathrm{ > CH_3 - C \equiv CH > CH \equiv CH}\) |
The molecule among the following that has the hybridization from left to right atoms is:
1.
2.
3.
4.
X and Y in the above-mentioned reaction are respectively:
1. X = 2–Butyne; Y = 3–Hexyne
2. X = 2-Butyne; Y = 2-Hexyne
3. X = 1-Butyne; Y = 2-Hexyne
4. X = 1-Butyne; Y = 3–Hexyne
For the above-given reaction, A (Predominantly) is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The order of decreasing reactivity towards an electrophilic reagent, for the following:
(i) Benzene (ii) Toluene
(iii) Chlorobenzene and
(iv) Phenol
would be:
1. (i) > (ii) > (iii) > (iv)
2. (ii) > (iv) > (i) > (iii)
3. (iv) > (iii) > (ii) > (i)
4. (iv) > (ii) > (i) > (iii)
Consider the following reaction,
The structure of the major reaction intermediate formed in the above reaction is-
| 1. | \(H_{3} C - \underset{\underset{D}{\left|\right.}}{C} H - \underset{\underset{CH_{3}}{\left|\right.}}{C} H - \overset{\circ}{C} H_{2}\) | 2. | \(H_{3} C - \underset{\underset{D}{\left|\right.}}{C} H - \underset{\underset{CH_{3}}{\left|\right.}}{\overset{\circ}{C}} - CH_{3}\) |
| 3. | \(H_{3} C - \underset{\underset{D}{\left|\right.}}{\overset{\circ}{C}} - \underset{\underset{CH_{3}}{\left|\right.}}{CH} - CH_{3}\) | 4. | \(H_{3} C - \underset{}{\overset{\circ}{C} H} - \underset{\underset{CH_{3}}{\left|\right.}}{CH} - CH_{3}\) |
Given below are two statements:
| Assertion (A): | The compound tetraene has the following structural formula. It is a cyclic and has conjugated 8π electrons system but it is not an aromatic compound. |
| Reason (R): | ( 4 n + 2 ) π electrons rule does not hold good for this compound and the ring is not planar. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | (A) is False but (R) is True. |
| Assertion (A): | Toluene on Friedel Crafts methylation gives o- and p- xylene. |
| Reason (R): | -group bonded to benzene ring increases electron density at o- and p- position. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True and (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | (A) is False but (R) is True. |
The molecular formula of diphenylmethane is C13H12. find out the number of structural isomers possible when one of the hydrogens is replaced by a chlorine atom:
| 1. | Four (4) | 2. | Eight (8) |
| 3. | Seven (7) | 4. | Six (6) |
The major product in the following reaction is:
1. CH3CD(Cl)CHD(I)
2. CH3CD2CH(Cl)(I)
3. CH3CD(I)CHD(Cl)
4. CH3C(I)(Cl)CHD2
Consider the following reaction,
The major product in the above reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The product A in the following reaction is:

| 1. |  |
2. |  |
| 3. |  |
4. |  |
| Assertion (A): | \(CH\equiv C-CH_2-CH=CH_2\) adds up HBr to give \(CH\equiv C-CH_2-CH-CH_3\\ ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~|\\ ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~Br \), while \(CH\equiv C-CH=CH_2\) adds HBr to give \(CH_2= C-CH=CH_2\\ ~~~~~~~~~~~~~~|\\ ~~~~~~~~~~~~~Br\) |
| Reason (R): | A double bond is more reactive than a triple bond towards an electrophilic \(\)addition reaction. |
| 1. | Both (A) and (R) are True, and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True, but (R) is not the correct explanation of (A). |
| 3. | Both (A) and (R) are False. |
| 4. | (A) is False, but (R) is True. |

| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
| 1. | n-Butane | 2. | Ethane |
| 3. | Methane | 4. | Ethene |
| Column-I | Column-II | ||
| a. | Non-benzenoid aromatic compound | (i) | Cyclooctatetraene |
| b. | Benzenoid aromatic compound | (ii) | Tropone |
| c. | Antiaromatic compound | (iii) | Naphthalene |
| d. | Non-aromatic compound | (iv) | 1,3-Cyclobutadiene |

| 1. | 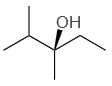 |
2. | 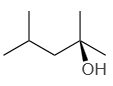 |
| 3. | 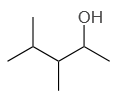 |
4. | 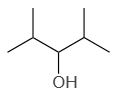 |
| I: |  |
| II: |  |