Physics-Section-A
1. A body of mass \(m\) is taken from the earth's surface to the height \(h\) equal to the radius of the earth, the increase in potential energy will be:
1. \(mgR\)
2. \(\frac{1}{2}~mgR\)
3. \(2 ~mgR\)
4. \(\frac{1}{4}~mgR\)
2. A uniform tube is shown in the figure which is open at one end and closed at the other. To enclose a column of air inside the tube, a pellet of mercury is introduced. If the length of air column at 27°C is 18 cm, at what temperature its length will be 21.6 cm?

1. 87°C
2. 91°C
3. 85°C
4. 97°C
3. A stick of length L mass M initially upright on a frictionless floor, starts falling, then
1. Center of mass will fall vertically down
2. Centre of mass will follow a circular path
3. Centre of mass will follow any curve path
4. All of these
4. When fluids are heated from the bottom, convection currents are produced because -
(1) Molecular motion of fluid becomes aligned
(2) Molecular collisions take place within the fluid
(3) Heated fluid becomes more dense than the cold fluid above it
(4) Heated fluid becomes less dense than the cold fluid above it
5. A particle of mass
\(m\) slides down a fixed smooth hemi-spherical bowl, starting from its rim. The normal reaction on the block when it reaches the lowest point is
\(N.\) Then:

| 1. |
\(N=mg\) |
2. |
\(N=2mg\) |
| 3. |
\(N=3mg\) |
4. |
\(N=5mg\) |
6. A block of iron of volume
\(V_0\) (in air) is taken to a depth
\(x\) under the sea (assume that the density of sea-water is constant). The volume of this block decreases by
\(\Delta V\) due to pressure of sea-water. Then,
\(\Delta V\) is proportional to:
| 1. |
\(x\) |
2. |
\(x^2\) |
| 3. |
\(x^3\) |
4. |
\(x^{-3}\) |
7. The force on a charge \(q\) in an electric field \(E\) is \(qE,\) while the force on the same charge \(q\) moving with a speed \(v\) perpendicular to a magnetic field \(B\) is \(qvB.\) Then, it can be concluded that \(E/B\) has dimensions of:
1. Electric charge
2. Electric current
3. Velocity
4. Acceleration
8. In which of the following processes does the internal energy of a gas remain constant?
1. Isothermal
2. Isochoric
3. Isobaric
4. Adiabatic
9. Consider the following figure representing the displacement of an object in one dimension.

Which of the following best represents the graph of acceleration versus time?
10. Two particles
\(A,B\) move along the periphery of a circle of radius
\(R,\) with the same uniform speed
\(u.\) Particle
\(A\) follows
\(B,\) a quarter of the circumference behind it. The acceleration of
\(A\) relative to
\(B\) is:

| 1. |
zero |
2. |
\(\dfrac{2u^2}{R}\) |
| 3. |
\(\dfrac{u^2}{\sqrt2R}\) |
4. |
\(\dfrac{\sqrt2u^2}{R}\) |
11. If the displacement equation of a particle be represented by , the particle executes
1. A uniform circular motion
2. A uniform elliptical motion
3. A S.H.M.
4 A rectilinear motion
12. A rod of mass \(M\) and length \(L\) is suspended vertically at its highest point. The rod is held so that it is horizontal and free to rotate about \({A}\) and then released. There is no friction anywhere.

The force exerted by the hinge at \({A},\) when the rod is at its lowest position is:
1. \(2~Mg\)
2. \(3~Mg\)
3. \(4~Mg\)
4. \(2.5~Mg\)
14. The magnitude of the vector
\(\widehat i+\widehat i\times\widehat j+(\widehat i\times\widehat j)\times\widehat i+((\widehat i\times\widehat j)\times\widehat i)\times\widehat j\):
1. \(1\)
2. \(\sqrt2\)
3. \(\sqrt3\)
4. \(2\)
Physics-Section-B
15. An object moves at a constant speed along a circular path in a horizontal \(XY\) plane with its centre at the origin. When the object is at \(x=-2~\text{m}\), its velocity is \(-(4~\text{m/s})\hat j\). What is the object's acceleration when it is at \(y = 2~\text{m}\)?
| 1. |
\(-(8~\text{m/s}^2)\hat j\) |
2. |
\(-(8~\text{m/s}^2)\hat i\) |
| 3. |
\(-(4~\text{m/s}^2)\hat j\) |
4. |
\(-(4~\text{m/s}^2)\hat i\) |
16. Which one of the following is not a vector quantity?
1. Velocity
2. Weight
3. Electric charge
4. Electric field
17. A simple pendulum of length
\(L\) is suspended from a point
\(O\) in a rocket which is ready to be launched from earth. The rocket takes off with an upward acceleration equal to
\(g.\) Immediately after take off, the bob of the pendulum is given a horizontal velocity so that it just completes a vertical circle. The bob's speed (relative to the rocket) at its highest point is
\(v_H,\) where:
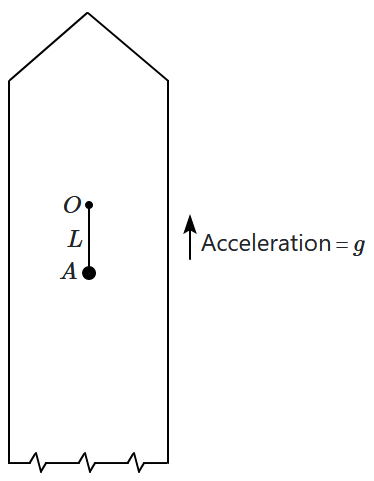
1.
\(v_H=\sqrt{gL}\)
2.
\(v_H=\sqrt{2gL}\)
3.
\(v_H=\sqrt{3gL}\)
4.
\(v_H=\sqrt{5gL}\)
18. If the unit of length is the distance between the earth and the sun, and the unit of time is
\(1\) year, then the speed of the earth around the sun is numerically equal to:
| 1. |
\(1\) |
2. |
\(2\) |
| 3. |
\(2\pi\) |
4. |
\(\dfrac1{2\pi}\) |
19. A boy of mass \(40\) kg is hanging from the horizontal branch of a tree. The tension in his arms is minimum when the angle between the arms is:
1. \(0^\circ\)
2. \(90^\circ\)
3. \(120^\circ\)
4. \(180^\circ\)
20. A particle moves along a straight line with its velocity \((v)\) varying as the square root of its displacement \((x)\text:\) \(v\propto\sqrt x\)
Then its acceleration varies as:
1. \(\dfrac{1}{\sqrt x}\)
2. \(x^{3/2}\)
3. \(x^{-3/2}\)
4. \(x^0\)
Chemistry-Section-A
21. What is the value of
\(\mathrm{K_{sp}}\) of
\(\mathrm {Ag_2CO_3 (s) }\) in water at 25º C for the following reaction;
\(\mathrm{Ag_2 CO_3 (s) \rightarrow 2Ag^+ (aq) + CO^{2-}_3 (aq)}\)
[Given:
\(\text R = 8.314 \text J\text K^{–1} \text {mol}^{–1}\) ;
\(\Delta \text G^\circ=+63.3~\text{kJ}\) ]
1.
\(3.2 \times 10^{26}\)
2.
\(8.0 \times 10^{-12}\)
3.
\(2.9 \times 10^{-3}\)
4.
\(7.9 \times 10^{-2}\)
22. 0.75 grams of a nitrogeneous compound is boiled with NaOH resulting in release of ammonia gas. The evolved ammonia is passed into 100 ml \(N\over 2\) H2SO4. The excess acid requires 90 ml \(N \over 3\)NaOH to neutralise. The percentage of nitrogen in the compound is:
1. 25
2. 37.33
3. 57.33
4. 75
23. In the preparation of
\(H_2O_2\), when 50%
\(H_2SO_4\) solution is electrolysed, the ion discharged at the anode is:
| 1. |
\(SO^{2-}_4\) |
2. |
\(OH^-\) |
| 3. |
\(HSO^-_4\) |
4. |
\(Cl^-\) |
24. An aqueous solution of NaHSO3 is-
1. Acidic
2. Slightly alkaline
3. Alkaline
4. Slightly Acidic
25. What is the mass of the precipitate formed when 50 mL of 16.9% solution of AgNO3 is mixed with 50 mL of 5.8% NaCl solution? (Ag = 107.8, N = 14, O = 16, Na= 23, Cl = 35.5)
1. 28 g
2. 3.5 g
3. 7 g
4. 14 g
26. The isostructural pairs among the following are:
| A. |
\( \mathrm{SO}_{4}^{2-} and \ \mathrm{CrO}_{4}^{2-}\) |
| B. |
\(\mathrm{SiCl}_{4} \ and \ \mathrm{TiCl}_{4}\) |
| C. |
\(\mathrm{NH}_{3} \ and \ \mathrm{NO}_{3}^{-} \) |
| D. |
\(\mathrm{BCl}_{3} \ and \ \mathrm{BrCl}_{3}\) |
1. C and D only
2. A and B only
3. A and C only
4. B and C only
27. Biochemical Oxygen Demand (BOD), is a measure of organic material present in water. BOD value less than 5 ppm indicates a water sample to be
1. Rich in dissolved oxygen
2. Poor in dissolved oxygen
3. Highly polluted
4. Not suitable for aquatic life
28. Identify the incorrect statement from the following:
| 1. |
The shapes of dxy, dyz, and dzx orbitals are similar to each other; and dx2 -y2 and dz2 are similar to each other. |
| 2. |
All the five 5d orbitals are different in size when compared to the respective 4d orbitals. |
| 3. |
All the five 4d orbitals have shapes similar to the respective 3d orbitals. |
| 4. |
In an atom, all the five 3d orbitals are equal in energy in free state. |
29. The thermal stability of these hydrides decreases in which of the following orders?
(1) CsH > RbH > KH > NaH > LiH
(2) KH > NaH > LiH > CsH > RbH
(3) NaH > LiH > KH > RbH > CsH
(4) LiH > NaH > KH > RbH > CsH
31. The partial pressure of oxygen in a flask containing 16 g and 32 g is-
1. 1/16 of total pressure
2. 1/2 of total pressure
3. 2/3 of total pressure
4. None of the above
32. Which of the following statement regarding is wrong.
(1) It is stable in acid medium
(2) It acts as oxidising as well as reducing agent
(3) It has zero dipole moment
(4) Pure is slightly acidic
33. Select the element (M) whose trihalides cannot be hydrolysed to form [M(H
2O)
6]
3+.
34. Elements in the same group have similar physical and chemical properties due to the same:
1. Electronic configuration
2. Valence electrons
3. Atomic size
4. Atomic mass
Chemistry-Section-B
35. Given below are pair of species. Which pair has the largest difference in sizes?
| 1. |
Na and K |
2. |
K and K+ |
| 3. |
Cl and Cl- |
4. |
Cl and Br |
36. In which shell of hydrogen atom, velocity of electron is the highest?
| 1. |
1st shell |
2. |
2nd shell |
| 3. |
3rd shell |
4. |
4th shell |
37. Match molecules in
List-I with the Corresponding shape in
List-II:
|
List-I
(Molecules) |
|
List-II
(Shape) |
| (a) |
NH3 |
(i) |
Square pyramidal |
| (b) |
ClF3 |
(ii) |
Trigonal bipyramidal |
| (c) |
PCl5 |
(iii) |
Trigonal pyramidal |
| (d) |
BrF5 |
(iv) |
T-shape |
Choose the correct answer from the options given below:
|
(a) |
(b) |
(c) |
(d) |
| 1. |
(ii) |
(iii) |
(iv) |
(i) |
| 2. |
(iii) |
(iv) |
(ii) |
(i) |
| 3. |
(iv) |
(iii) |
(i) |
(ii) |
| 4. |
(iii) |
(iv) |
(i) |
(ii) |
38. The mean free path of gas A, with molecular diameter equal to 4 Å, contained in a vessel, at a pressure of torr, is 6990 cm. The vessel is evacuated and then filled with gas B, with molecular diameter, equal to 2 Å, at a pressure of torr, the temperature remaining the same. The mean free path of gas B will be
(A) 28 cm
(B) 280 cm
(C) 7 cm
(D) 14 cm
39. Given the following reaction:
\(\begin{aligned} & \frac{1}{2} \mathrm{H}_2+\frac{1}{2} \mathrm{Cl}_2 \rightarrow \mathrm{HCl} \\ & \Delta H_f(\mathrm{HCl})=-93 \mathrm{~kJ} / \mathrm{mol} \\ & \text { B.E }\left(\mathrm{H}_2\right)=434 \mathrm{~kJ} / \mathrm{mol}, \text { B.E }\left(\mathrm{Cl}_2\right)=242 \mathrm{~kJ} / \mathrm{mol} \end{aligned}\)
The bond dissociation energy of HCl in the above reaction will be:
1. 232 kJ/mol
2. 331 kJ/mol
3. 431 kJ/mol
4. 530 kJ/mol
40. The molarity of a solution obtained by mixing 750 mL of 0.5M HCl with 250 ml of 2M HCl will be:
1. 1.75 M
2. 0.975 M
3. 0.875 M
4. 1.00 M
Biology-I-Section-A
41. Consider the given two statements:
| I: |
In a test cross, the allele the individual in question [one that expresses the dominant phenotype] passes on, determines the phenotype of the offspring. |
| II: |
The homozygous recessive individual can only pass on recessive alleles. |
| 1. |
Both I and II are correct and II explains I |
| 2. |
Both I and II are correct but II does not explain I |
| 3. |
I is correct but II is incorrect |
| 4. |
I is incorrect but II is correct |
42. Anthropogenic extinction is called
1. Fifth mass extinction
2. Fouth mass extinction
3. Sixth mass extinction
4. Seventh mass extinction
43. State true (T) or false (F) for the following statements and select the correct option.
A. In secondary productivity, no new organic matter is formed.
B. Detritus food chain begins with detritus.
|
A |
B |
| 1. |
T |
T |
| 2. |
T |
F |
| 3. |
F |
T |
| 4. |
F |
F |
44. The gynoecium with many pistils that are fused together will best be described as:
1. monocarpellary, apocarpous
2. multicarpellary, syncarpous
3. multicarpellary, synandrous
4. monocarpellary, apocarpous
45. Which of the following ion is important for the activity of Pyruvate dehydrogenase?
(1) Zn++
(2) Mn+
(3) Mg++
(4) Ca++
46. Match each item in Column I with one item in Column II and choose the correct answer from the code given below:
| A. |
Air [Prevention and Control of Pollution] Act |
a. |
1981 |
| B. |
Water [Prevention and Control of Pollution] Act |
b. |
1974 |
| C. |
Environment [Protection] Act |
c. |
1986 |
| D. |
Montreal Protocol |
d. |
1987 |
Codes:
|
A |
B |
C |
D |
| 1. |
a |
b |
c |
d |
| 2. |
b |
a |
d |
c |
| 3. |
d |
c |
b |
a |
| 4. |
c |
d |
a |
b |
47. The elements which are not actively mobilizable or immobilized within the plants. The deficiency symptom is observed in
1. Both in young and old parts
2. Only in young parts
3. First in old parts
4. First in young parts
48. Which of the following statement is incorrect?
1. The greater the BOD of waste water, more is its polluting potential
2. Baculoviruses are pathogens that attack several insects and plants
3. Trichoderma species are effective biocontrol agents of several plant pathogens
4. Sporeine was first bioinsecticide
49. What would be the chromosome number in an endosperm cell of garden pea [
Pisum sativum]?
50. The phenomenon by which the undividing parenchyma cells start to divide mitotically during plant tissue culture is called as :
1. Differentiation
2. Dedifferentiation
3. Redifferentiation
4. Secondary growth
51. Exotic species:
| A. |
These are new species entering a geographical area. |
| B. |
They may cause the disappearance of the native species. |
| C. |
They have a huge impact on the world's highly threatened island ecosystem. |
| D. |
They are considered as the major cause of the extinction of the species. |
Choose the correct answer from the options given below:
1. A and B only
2. A and C only
3. A, B and C only
4. A, C and D only
52. Regarding methodologies used in sequencing human genome:
| I: |
Identifying all the genes that are expressed as RNA is referred to as Expressed Sequence Tags. |
| II: |
The blind approach of simply sequencing the whole set of genome that contained all the coding and non-coding sequence, and later assigning different regions in the sequence with functions is referred to as Sequence Annotation. |
1. Only
I is correct
2. Only
II is correct
3. Both
I and
II are correct
4. Both
I and
II are incorrect
53. Consider the two statements:
| Assertion (A): |
The primary acceptor of carbon dioxide in C3 plants is a 2-carbon compound. |
| Reason (R): |
The first product of carbon dioxide fixation in these plants is a C3 acid. |
| 1. |
(A) is True but (R) is False |
| 2. |
Both (A) and (R) are True and (R) explains (A) |
| 3. |
Both (A) and (R) are True but (R) does not explain (A) |
| 4. |
(A) is False but (R) is True |
54. Consider the given two statements:
| Assertion (A): |
Inbreeding increases the chances of offspring being affected by recessive traits. |
| Reason (R): |
Inbreeding increases the proportion of homozygous individuals in a population. |
| 1. |
Both (A) and (R) are True and (R) correctly explains the (A). |
| 2. |
Both (A) and (R) are True but (R) does not correctly explain the (A). |
| 3. |
(A) is True but (R) is False. |
| 4. |
Both (A) and (R) are False. |
Biology-I-Section-B
55. Match each item in Column I with one in Column II and select the correct match from the codes given:
|
Column I |
|
Column II |
| A |
Ribosome |
P |
Acid hydrolases |
| B |
Lysosome |
Q |
Protein synthesis |
| C |
Centrosome |
R |
Chromatin subunit |
| D |
Nucleosome |
S |
Cell division |
Codes:
|
A |
B |
C |
D |
| 1. |
Q |
P |
S |
R |
| 2. |
P |
Q |
R |
S |
| 3. |
P |
Q |
S |
R |
| 4. |
Q |
P |
R |
S |
56. Match each item in Column I with one in Column II and select the correct match from the codes given:
|
Column I
[Pteridophyte class] |
|
Column II
[Example] |
| A |
Psilopsida |
P |
Psilotum |
| B |
Lycopsida |
Q |
Adiantum |
| C |
Sphenopsida |
R |
Selaginella |
| D |
Pteropsida |
S |
Equisetum |
Codes:
|
A |
B |
C |
D |
| 1. |
P |
R |
Q |
S |
| 2. |
P |
R |
S |
Q |
| 3. |
P |
S |
R |
Q |
| 4. |
Q |
S |
R |
P |
57. Match each item in Column I with one in Column II and select the correct match from the codes given:
|
Column I
[Placentation] |
|
Column II
[Example] |
| A |
Axile |
P |
Mustard |
| B |
Parietal |
Q |
Dianthus |
| C |
Free central |
R |
Lemon |
| D |
Basal |
S |
Marigold |
Codes:
|
A |
B |
C |
D |
| 1. |
R |
P |
Q |
S |
| 2. |
P |
R |
Q |
S |
| 3. |
P |
Q |
R |
S |
| 4. |
R |
P |
S |
Q |
58. The scientific names, given to each organism, ensure that
| I: |
each organism has only one name. |
| II: |
such a name has not been used for any other known organism. |
1. Both
I and
II are incorrect
2. Only
I is correct
3. Only
II is correct
4. Both
I and
II are correct
59. In the five kingdom classification, the members of Kingdom Animalia:
| I: |
are all heterotrophs |
| II: |
are all multicellular where cells lack a cell wall |
1. Only I is correct
2. Only II is correct
3. Both I and II are correct
4. Both I and II are incorrect
60. The cells of parenchyma are usually
1. Diametric
2. Isodiametric
3. Pentagonal
4. Orthorhombic
Biology-II-Section-A
61. Which of the following is true about pituitary gland?
1. It is known as master gland
2. It is responsible for pigmentation of skin
3. It stimulates both milk synthesis and its ejection
4. All of the above
62. Which of the following is a powerful vasoconstrictor that increases the glomerular blood pressure and thereby the GFR?
1. Renin
2. Angiotensin-II
3. Aldosterone
4. ANF
63. Oral contraceptive pills contain
1. FSH and LH hormones
2. Progestogen and estrogen combination
3. Prolactin
4. Mifepristone
64. Read the given statements.
| Statement A: |
Evolution is a directed process in the sense of determinism. |
| Statement B: |
Evolution is a stochastic process based on chance events in nature and chance mutation in the organisms. |
In the light of above given statements, select the correct option
| 1. |
Both statements A and B are correct |
| 2. |
Both statements A and B are incorrect |
| 3. |
Only statement A is correct |
| 4. |
Only Statement B is correct |
65. Which of the following is best for the effective treatment of a disease?
1. Early diagnosis only
2. Understanding the pathophysiology only
3. Diagnosing only after the symptoms have manifested
4. Early diagnosis and understanding its pathophysiology
66. Consider the given two statements:
| Assertion (A): |
Neurons are excitable cells. |
| Reason (R): |
The membrane of neurons is polarised. |
| 1. |
Both (A) and (R) are True and (R) correctly explains the (A). |
| 2. |
Both (A) and (R) are True but (R) does not correctly explain the (A). |
| 3. |
(A) is True but (R) is False. |
| 4. |
Both (A) and (R) are False. |
67. Match each item in Column I with one in Column II and select the correct match from the codes given:
|
Column I
[Blood type] |
|
Column II
[Can receive blood from] |
| A |
A |
P |
A and O |
| B |
B |
Q |
Only O |
| C |
AB |
R |
A, B, AB and O |
| D |
O |
S |
B and O |
Codes:
|
A |
B |
C |
D |
| 1. |
S |
P |
Q |
R |
| 2. |
P |
S |
R |
Q |
| 3. |
P |
Q |
S |
R |
| 4. |
S |
P |
R |
Q |
68. Match List I with List II
| List I |
List II |
| A. Chitin |
I. Polymer of fructose |
| B. Lecithin |
II. Protein |
| C. Collagen |
III. Polysaccharide |
| D. Inulin |
IV. Phospholipid |
Choose the correct answer from the options given below:
1. A-III, B-II, C-I, D-IV
2. A-III, B-I, C-II, D-IV
3. A-IV, B-III, C-I, D-II
4. A-III, B-IV, C-II, D-I
69. Where is the antigen binding site present in an antibody molecule?
| 1. |
between the two light chains |
| 2. |
between the two heavy chains |
| 3. |
between the light and the heavy chains |
| 4. |
at the very end of the antibody molecules [at their C terminus] |
70. Which of the following is not a synovial joint?
(1) Between axis and Atlas
(2) Between carpals
(3) Between vertebrae
(4) Metacarpal and carpal of thumb
71. The amount of CO2 that can diffuse through the diffusion membrane per unit difference in partial pressure is much higher as compared to that of O2. This is because:
| 1. |
Solubility coefficient of CO2 is higher |
| 2. |
Solubility coefficient of CO2 is lesser |
| 3. |
Amount of gases in blood is independent of partial pressures of the gases in the atmosphere |
| 4. |
Arterial blood contains more O2 than CO2 |
72. Goblet cells, present in alimentary canal, are used in:
1. Absorption
2. Protection
3. Secretion of mucus
4. Contraction
73. Agrobacterium tumefaciens infects the plant through its:
| 1. |
Chromosomal DNA |
2. |
Complementary DNA |
| 3. |
F plasmid |
4. |
Ti plasmid |
74. Spermatogenesis starts at the age of puberty due to significant increase in the secretion of:
| 1. |
LH by anterior pituitary |
2. |
Inhibin by Sertoli cells |
| 3. |
GnRH by hypothalamus |
4. |
Androgens by Leydig cells |
Biology-II-Section-B
75. Consider the pie chart given below w.r.t. average composition of substances in cells.

Identify the component A, B, C, D and E. Select the correct option.
| 1. |
A= Water ; B=Carbohydrates ; C=Proteins ; D=Nucleic acids ; E=Lipids |
| 2. |
A=Proteins ; B=Water ; C=Carbohydrates ; D=Lipids ; E=Nucleic acids |
| 3. |
A=Water ; B=Proteins ; C=Nucleic acids ; D=Carbohydrates ; E=Lipids |
| 4. |
A=Proteins ; B=Carbohydrates ; C=Nucleic acids ;D=Lipids E=Nucleic |
76. Identify the incorrect statement regarding carbonic anhydrase:
| 1. |
RBCs contain a very high concentration of the enzyme. |
| 2. |
It is not present at all in the plasma. |
| 3. |
It assists rapid inter-conversion of carbon dioxide and water into carbonic acid, protons and bicarbonate ions. |
| 4. |
The active site of the enzyme contains a zinc ion. |
77. Match each item in
Column-I with the one in
Column-II and select the correct match from the codes given:
|
Column-I |
|
Column-II |
| A. |
Cephalochordata |
P. |
Swim bladder |
| B. |
Cyclostomata |
Q. |
Notochord – head to tail |
| C. |
Chondrichthyes |
R. |
Males bear claspers on pelvic fins |
| D. |
Osteichthyes |
S. |
Marine but migrate to freshwater for spawning |
Codes:
|
A |
B |
C |
D |
| 1. |
S |
Q |
P |
R |
| 2. |
P |
Q |
R |
S |
| 3. |
Q |
S |
R |
P |
| 4. |
R |
P |
S |
Q |
78. Consider the two statements:
| Statement I: |
When swallowing, the soft palate would have to move downward in order to prevent food from entering the lungs. |
| Statement II: |
When swallowing the epiglottis allows the food to enter the larynx. |
1. Statement I is correct: Statement II is incorrect
2. Statement I is incorrect: Statement II is correct
3. Statement I is correct: Statement II is correct
4. Statement I is incorrect: Statement II is incorrect
79. The risk of erythroblastosis foetalis exists when:
| 1. |
an Rh positive mother is carrying an Rh negative foetus |
| 2. |
an Rh negative mother is carrying an Rh positive foetus |
| 3. |
an Rh positive mother is carrying an Rh positive foetus |
| 4. |
an Rh negative mother is carrying an Rh negative foetus |
80. Which of the following is false?
(1) Adipose tissue is located mainly below skin
(2) Tendon consists of white fibrous tissue
(3) Tendon connects a bone with another bone
(4) Ligaments connect a bone with another bone
*If above link doesn't work, please go to test link from where you got the pdf and fill OMR from there
CLICK HERE to get FREE ACCESS for 2 days of ANY NEETprep course











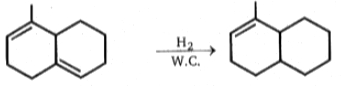
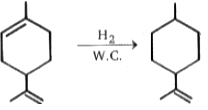 (w.c. = wilkinsons catalyst)
(w.c. = wilkinsons catalyst)![]() (w.c. = wilkinsons catalyst)
(w.c. = wilkinsons catalyst)