The compound that will not form a yellow precipitate on heating with an alkaline solution of iodine is:
1. CH3CH(OH)CH3
2. CH3CH2CH(OH)CH3
3. CH3OH
4. CH3CH2OH
Glycerol on oxidation with conc HNO3 gives:
1. tartronic acid
2. mesoxalic acid
3. oxalic acid
4. glyceric acid
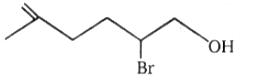
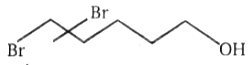
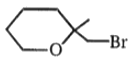
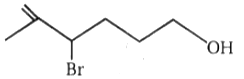
The number of moles of HI
consumed by the given compound is:
| 1. | 1 | 2. | 2 |
| 3. | 3 | 4. | 4 |
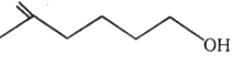

1. 
2. 
3. 
4. 
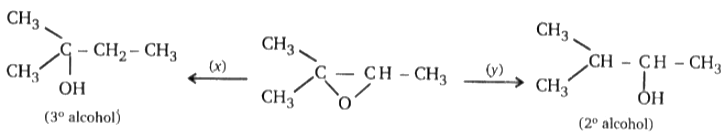
Find missing reagents.
1. x = LiAlH4, y = NaBH4
2. x = LiAIH4 / AlCl3, y = LiAlH4
3. x = LiAIH4, Y = LiAIH4 / AlCl3
4. x = H2 / Ni, y = H2 / Pt
Which are not cleaved by HIO4?
I : glycerol
II : glycol
III: 1, 3-propanediol
IV: methoxy-2-propanol
1. I, II, III, IV
2. I, II
3. II, III
4. III, IV

1. Dichloromethyl cation \((^{+}CHCl_{2})\)
2. Formyl cation \((^{+}CHO)\)
3. Dichloromethyl anion \((^{-}CHCl_{2})\)
4. Dichlorocarbene (:CCl2)
The compound, among the following, that will not be soluble in sodium hydrogen carbonate is:
| 1. | 2, 4, 6-Trinitrophenol | 2. | Benzoic acid |
| 3. | o-Nitrophenol | 4. | Benezenesulphonic acid |
1-propanol and 2-propanol can be best distinguished
by:
1. Oxidation with alkaline KMnO4 followed by
reaction with Fehling solution.
2. Oxidation with acidic dichromate followed by
reaction with Fehling solution
3. Oxidation by heating with copper followed by
reaction with Fehling solution.
4. Oxidation with conc. H2SO4
followed by reaction with Fehling solution
The product ‘A’ is
(A) and (B) are respectively :