A pair that represents chain isomer is:
1. CH3CHCl2 and ClCH2CH2Cl
2. Propyl alcohol and Isopropyl alcohol
3. 2-Methylbutane and Neopentane
4. Diethyl ether and Dipropylether
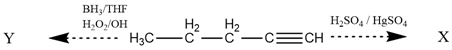 . Where X and Y are
. Where X and Y are
\(\xleftarrow{\mathrm {BH_3/THF\\{H_2O_2/OH}}}~\mathrm {H_3C-CH_2-CH_2-CH\xrightarrow{H_2SO_4/H_2SO_4}}~\mathrm X\\\)
1. Positional isomers
2. Functional isomers
3. Metamers
4. Tautomers
Which of the following may exist as enantiomers?
| 1. |  |
| 2. | \(\mathrm{CH}_2=\mathrm{CHCH}_2 \mathrm{CH}_2 \mathrm{CH}_3 \) |
| 3. |  |
| 4. |  |
The transition in the He+ ion in the balmer series that would have the same wave number as the first Lyman line in the hydrogen spectrum is-
| 1. | \(2 \rightarrow1\) | 2. | \(5 \rightarrow3\) |
| 3. | \(4 \rightarrow2\) | 4. | \(6 \rightarrow4\) |
A cricket ball of 0.5 kg is moving with a velocity of 100 ms-1. The wavelength associated with its motion is:
1. 1/100cm
2. 6.6 x 10-34m
3. 1.32 x 10-35m
4. 6.6 x 10-28m
The maximum wavelength in the Lyman series of He+ ion is-
1. 3R
2. 1/3R
3. 1/R
4. 2R
The IUPAC name of 
1. 2-Phenylpropan-1-ol
2. 2-Phenylpropan-3-ol
3. 1-(2-Hydroxy-1-methylethyl)benzene
4. 1-((Hydroxymethyl)ethyl)benzene
The IUPAC name of 
1. 4-Bromo benzenamine
2. 4-Amino-1-bromobenzene
3. 4-Bromo benzenamide
4. 1-Bromo benzencarboxamide
The-I effect is shown by:
1. -COOH
2. -CH3
3. -CH3CH2
4. -CHR2
Which of the following series of transitions in the spectrum of hydrogen atoms falls in the visible region?
1. Brackett series
2. Lyman series
3. Balmer series
4. Paschen series
Which orbital has the maximum number of total nodes?
1. 5s
2. 5p
3. 5d
4. All have the same number of nodes.
represents: (for Schrodinger wave mechanical model)
1. Amplitude of electron wave
2. Probability density of electron
3. Total probability of finding electron around nucleus
4. Orbit
The incorrect statement about the nodal plane among the following is:
| 1. | A plane on which there is a zero probability of finding an electron. |
| 2. | A plane on which there is maximum probability that the electron will be found. |
| 3. | ψ2 is zero at nodal plane. |
| 4. | None of the above. |
Which of the following carboxylic acids will be the most acidic?
1.
2.
3.
4.
A positive charge on the molecule shown would have greater stability than a positive charge on a straight-chain alkane version of the same molecule. What property most explains this effect?
1. Steric hindrane
2. Nitrogen electronegativity
3. Induction
4. Conjugation