Physics-Section-A
1. If force (\(F\)), velocity (\(\mathrm{v}\)), and time (\(T\)) are taken as fundamental units, the dimensions of mass will be:
| 1. |
\([FvT^{-1}]\) |
2. |
\([FvT^{-2}]\) |
| 3. |
\([Fv^{-1}T^{-1}]\) |
4. |
\([Fv^{-1}T]\) |
2. Two pendulums of length
\(121~\text{cm}\) and
\(100~\text{cm}\) start vibrating in phase. At some instant, the two are at their mean position in the same phase. The minimum number of vibrations of the shorter pendulum after which the two are again in phase at the mean position is:
| 1. |
\(8\) |
2. |
\(11\) |
| 3. |
\(9\) |
4. |
\(10\) |
3. Two particles
\(A\),
\(B\) are projected simultaneously from the base of a triangle
\(ABC\). Particle
\(A\) is projected from vertex
\(A\) along
\(AC,\) and particle
\(B\) is projected from vertex
\(B\) along
\(BC\). Their respective velocities are
\(v_A\) &
\(v_B\) and they move with uniform velocities. For the particles to collide:

| 1. |
\(v_A~\text{cos}A=v_B~\text{cos}B\) |
| 2. |
\(v_A~\text{sin}A=v_B~\text{sin}B\) |
| 3. |
\(\dfrac{v_A}{\text{sin}A}=\dfrac{v_B}{\text{sin}B}\) |
| 4. |
\(v_A~\text{tan}A=v_B~\text{tan}B\) |
4. One mole of an ideal gas goes from an initial state \(A\) to the final state \(B\) with two processes. It first undergoes isothermal expansion from volume \(V\) to \(3V\) and then its volume is reduced from \(3V\) to \(V\) at constant pressure. The correct \((P-V)\) diagram representing the two processes is:
5. Consider a chessboard, as shown in the figure, where the width of each square is
\(2~\text{cm}.\) Assume that all pieces are kept at the centres of the squares, before and after a move. Also, assume that each single move takes
\(2~\text{s}\) – irrespective of however many squares (distance) are taken.

What is the maximum possible displacement of a rook
\(\Large(♖)\) in a single move?
Note: A rook can move any number of squares along only
\(x\) or only
\(y\) (positive or negative) direction.
| 1. |
\(14~\text{cm}\) |
2. |
\(16~\text{cm}\) |
| 3. |
\(18~\text{cm}\) |
4. |
\(16\sqrt2~\text{cm}\) |
6. A wedge-shaped vessel holds water. As one moves downward along
\(OB\) or
\(AB\) pressure changes. The increase in pressure per unit distance traversed:

| 1. |
is more along \(AB\) compared to \(OB\) |
| 2. |
is more along \(OB\) compared to \(AB\) |
| 3. |
is the same along \(AB\) or \(OB\) |
| 4. |
is zero along \(AB,\) but non-zero along \(OB\) |
7. A block of mass
\(m\) is placed on an incline, where the coefficient of friction is
\(\mu.\) The block is observed to be at rest. The force of friction on the block equals:

1.
\(mg~\text{cos}\theta\)
2.
\(mg~\text{sin}\theta\)
3.
\(\mu mg~\text{cos}\theta~\)
4.
\(\mu mg~\text{sin}\theta~\)
8. A particle moves from a point \(\left(\right. - 2 \hat{i} + 5 \hat{j} \left.\right)\) to \(\left(\right. 4 \hat{j} + 3 \hat{k} \left.\right)\) when a force of \(\left(\right. 4 \hat{i} + 3 \hat{j} \left.\right)\) \(\text{N}\) is applied. How much work has been done by the force?
| 1. |
\(8\) J |
2. |
\(11\) J |
| 3. |
\(5\) J |
4. |
\(2\) J |
9. A black body is at a temperature of \(5760~\text{K}.\) The energy of radiation emitted by the body at wavelength \(250~\text{nm}\) is \(U_1,\) at wavelength \(500~\text{nm}\) is \(U_2\) and that at \(1000~\text{nm}\) is \(U_3.\) Wien’s constant, \(b=2.88 \times 10^6~ \text{nm-K}.\) Which of the following is correct?
1. \({U}_3 =0 \)
2. \({U}_1 >{U}_2 \)
3. \({U}_2 >{U}_1 \)
4. \({U}_1 =0\)
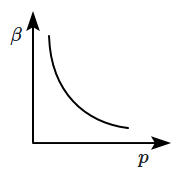
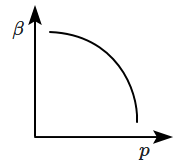
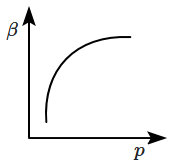
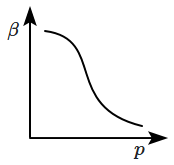
10. Which of the following graphs correctly represents the variation of
\(\beta=-\dfrac{1}{V}\dfrac{dV}{dp} \) with
\(p \) for an ideal gas at constant temperature?
11. Suppose the Earth suddenly contracts such that the duration of the new day is \(24/n^2\). If the original radius is \(r\), then the new radius assuming that the mass of Earth remains the same will be:
1. \(r/n^2\)
2. \(nr\)
3. \(r/n\)
4. \(2nr\)
12. Assuming the earth to be a sphere of uniform density, its acceleration due to gravity acting on a body:
| 1. |
increases with increasing altitude. |
| 2. |
increases with increasing depth. |
| 3. |
is independent of the mass of the earth. |
| 4. |
is independent of the mass of the body. |
13. Two wires of equal lengths
\((L)\) and cross-section
\((A)\) but different Young's moduli
\(Y_1\) and
\(Y_2\) are joined as shown. The total elongation of the composite wire, if a force
\(F\) is applied at the bottom, is:

| 1. |
\(\Large\frac{F}{AY_1}+\frac{F}{AY_2}\) |
2. |
\(\Large\frac{FY_1}{A}+\frac{FY_2}{A}\) |
| 3. |
\(\Large\frac{FL}{AY_1}+\frac{FL}{AY_2}\) |
4. |
\(\Large\frac{FL}{A\Big(\frac{Y_1+Y_2}{2}\Big)}\) |
14. A force \(F = (20 + 10 y)\) acts on a particle in the \(y\)-direction where \(F\) is in Newton and \(y\) is in metre. The work done by this force to move the particle from \(y =0\) to \(y =1~\text m\) is:
1. \(20~\text{J}\)
2. \(30~\text{J}\)
3. \(5~\text{J}\)
4. \(25~\text{J}\)
Physics-Section-B
15. A particle moving initially with a speed of
\(3~\text{m/s}\) around a circle accelerates so as to increase its speed by
\(3~\text{m/s}\) every half-revolution. If its initial kinetic energy is
\(E,\) its kinetic energy after a complete revolution will be:
1.
\(2E\)
2.
\(3E\)
3.
\(9E\)
4.
\(16E\)
16. A ball (mass : \(m\)) that is dropped from a height \(h\) above the ground, rebounds elastically and rises to the same level. The average force acting on this ball during the entire motion is:
1. \(mg\)
2. \(2mg\)
3. \(\dfrac{mg}{2}\)
4. zero
17. The velocity of a particle depends upon the time
\(t\) according to the equation:
\({v}{=}\sqrt{ab}{+}{bt}{+}\frac{c}{{d}{+}{t}}\)
The physical quantities are represented by
\(a,b,c\) and
\(d\) are in the order:
| 1. |
distance, distance, acceleration, time |
| 2. |
distance acceleration, distance, time |
| 3. |
acceleration, distance, distance, time |
| 4. |
none of the above |
18. If \(\vec F=2\hat i+\hat j-\hat k\) and \(\vec r=3\hat i+2\hat j-2\hat k,\) then the scalar and vector products of \(\vec F\) and \(\vec r\) have the magnitudes, respectively, as:
1. \(5, ~\sqrt3\)
2. \(4,~ \sqrt5\)
3. \(10, ~\sqrt2\)
4. \(10,~2\)
19. The velocity-time graph of a particle, moving along a straight time, is shown in the figure. The curve, when plotted, takes the form of a 'circle'. The magnitude of the average acceleration of the particle is:

| 1. |
\(1\) m/s2 |
| 2. |
\(2\) m/s2 |
| 3. |
less than \(1\) m/s2 |
| 4. |
greater than \(2\) m/s2 |
20. A particle is thrown with a velocity of \(\mathrm{u}\) m/s. If it passes through A and B as shown in the figure at time \(t_1=1~\text{s}\) and \(t_2=3~\text{s}\), then the value of \(\mathrm{h}\) is: ( Take \(g=10~\text{m/s}^{2}\))

| 1. |
\(15~\text{m}\) |
2. |
\(10~\text{m}\) |
| 3. |
\(30~\text{m}\) |
4. |
\(20~\text{m}\) |
Chemistry-Section-A
21. The oxidation states of two S-atoms in are:
1. +2 and +4
2. +3 and -2
3. +4 and -2
4. +6 and -2
22. Match the mass of elements given in Column I with the number of moles given in Column II and mark the appropriate choice:
|
Column I |
|
Column II |
| (A) |
28 g of He |
(i) |
2 moles |
| (B) |
46 g of Na |
(ii) |
7 moles |
| (C) |
60 g of Ca |
(iii) |
1 mole |
| (D) |
27 g of Al |
(iv) |
1.5 moles |
1. (A)→(iv), (B)→(iii), (C)→(ii), (D)→(i)
2. (A)→(i), (B)→(iii), (C)→(ii), (D)→(iv)
3. (A)→(iii), (B)→(ii), (C)→(i), (D)→(iv)
4. (A)→(ii), (B)→(i), (C)→(iv), (D)→(iii)
23. A ball weighing 10 g is moving with a velocity of 90 ms–1. If the uncertainty in its velocity is 5 %, then the uncertainty in its position is X × 10–33 m. The value of X will be :
[Given : h = 6.63 × 10–34 Js]
1. 1.17
2. 1.37
3. 1.67
4. 1.97
24. Given below are two statements:
| Assertion (A): |
Among halogens, chlorine has the highest negative electron gain enthalpy value. |
| Reason (R): |
The ionic radius of Cl- is 184 pm. |
| 1. |
Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. |
Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. |
(A) is True but (R) is False. |
| 4. |
(A) is False but (R) is True. |
25. The standard Gibbs energy change at 300 K for the reaction is 2494.2 J. At a given time, the composition of the reaction mixture is . The reaction proceeds in the:[assume R=8.314 J/K/mol; e-1 = 0.37]
| 1. |
Forward direction because Q > KC |
| 2. |
Reverse direction because Q > KC |
| 3. |
Forward direction because Q < KC |
| 4. |
Reverse direction because Q < KC |
26. An ionic compound is dissolved simultaneously in heavy water and ordinary water, its solubility is –
1. Larger in heavy water
2. Smaller in heavy water
3. Solubility is same in both waters
4. Smaller in simple water
27. The compressibility factor for nitrogen at 330 K and 800 atm is 1.90 and at 570 K and 200 atm is 1.10. A certain mass of occupies a volume of at 330 K and 800 atm. Calculate volume occupied by same quantity of gas at 570 K and 200 atm:
1. 1 L
2. 2 L
3. 3 L
4. 4 L
28. Which isomers of xylene would produce only a single mononitration product on treatment with HNO
3/H
2SO
4 ?

1. Only
I
2. Only
II
3. Both
I and
II
4. Neither
I nor
II
29. The incorrect statement among the following is:
1. All alkali metals are silvery white.
2. The density of potassium is less than that of sodium.
3. Compounds of group-1 elements are diamagnetic.
4. The melting point of group-1 elements increases down the group.
30. Based on the hyperconjugation effect described above,
identify the compound in which the double bond is the longest:
1. CH3- CH = CH - CH3
2. (CH3)3 C-CH = CH2
3. CH3-CH = CH-CH(CH3)2
4. CH2 = CH- CH2 -CH3
31. Which of the following species is not stable?
| 1. |
|
2. |
|
| 3. |
|
4. |
|
32. During the change of O2 to , the incoming electron that goes to the orbital is:
| 1. |
\(\sigma _{2pz}^{*}\) |
2. |
π2py |
| 3. |
\(\sigma _{2pz}\) |
4. |
π*2px |
33. A biosphere is composed of
1. Living organisms
2. Living organisms + Lithosphere
3. Living organisms + lithosphere + atmosphere
4. Living organisms + lithosphere + atmosphere + Hydrosphere
34. Five moles of an ideal gas at 1 bar and 298 K are expanded into a vacuum till the volume doubles. The work done is:
1. –RT ln V2/V1
2. CV(T2 – T1)
3. zero
4. – RT(V2 – V1)
Chemistry-Section-B
35. The ratio of radii of 2
nd & 3
rd Bohr’s orbit of H-atom is:
1. 2 : 3
2. 3 : 2
3. 4 : 9
4. 9 : 4
36. How many molecules among the following exhibit a non-zero dipole moment?
\(BeF_2 , BF_3 , H_2O , NH_3 , HCl , CCl_4 \)
1. Four (4)
2. Six (6)
3. Zero (0)
4. Three (3)
37. Which of the following options correctly describes the free expansion of an ideal gas under adiabatic conditions?
1. \(\mathrm{{q} \neq 0, ~~ \Delta {T}=0, ~~ {~W}=0} \)
2. \(\mathrm{{q}=0, ~~ \Delta {T}=0, ~~ {~W}=0} \)
3. \(\mathrm{{q}=0, ~~ \Delta {T}<0, ~~ {~W} \neq 0} \)
4. \(\mathrm{{q}=0, ~~ \Delta {T} \neq 0, ~~ {~W}=0} \)
38. 120 g of an ideal gas of molecular weight 40 g mole–1 are confined to a volume of 20 L at 400 K. The pressure of the gas is-
( )
1. 3.90 atm
2. 4.92 atm
3. 6.02 atm
4. 2.96 atm
39. Consider the following reaction.
Na2O + H2O → A
Cl2O7 + H2O → B
The product A and B are respectively:
1. A is NaOH; and B is HOCl
2. A is NaH; and B is HOCl
3. A is NaH; and B is HClO2
4. A is NaOH; and B is HClO4
40. What is the molality of a solution containing 20% (mass/mass) KI in water?
(molar mass of KI = 166 g mol–1)
1. 1.51
2. 7.35
3. 4.08
4. 2.48
Biology-I-Section-A
41. The mean annual precipitation is the most variable in:
1. Tropical forest
2. Desert
3. Grassland
4. Arctic tundra
42. Which PGR promotes flowering in pineapples, induces parthenocarpy in tomatoes and also used as herbicides?
| 1. |
Auxins |
2. |
Cytokinins |
| 3. |
Gibberellins |
4. |
Ethylene |
43. The most diverse group of organisms amongst the following are:
1. Mosses
2. Algae
3. Ferns and allies
4. Lichens
44. The phenotypic and genotypic ratios are identical in:
| I: |
F1 progeny of a monohybrid cross with complete dominance |
| II: |
F2 progeny of a monohybrid cross with incomplete dominance |
1. Only
I is correct
2. Only
II is correct
3. Both
I and
II are correct
4. Both
I and
II are incorrect
45. Identify the plant, anecdotally known as the "terror of Bengal" due to its invasive growth tendencies, that was introduced into India as an ornamental plant:
1. Parthenium hysterophorus
2. Lantana camara
3. Eicchornia crassipes
4. Acacia dealbata
46. Consider the two statements:
| Assertion (A): |
Except for plants in shade or in dense forests, light is rarely a limiting factor for photosynthesis in nature. |
| Reason (R): |
Increase in incident light beyond a point causes the breakdown of chlorophyll and a decrease in photosynthesis. |
| 1. |
(A) is True but (R) is False |
| 2. |
Both (A) and (R) are True and (R) explains (A) |
| 3. |
Both (A) and (R) are True but (R) does not explain (A) |
| 4. |
(A) is False but (R) is True |
47. Which of the following features characterizes humus?
1. Easily broken down by microbes
2. Rapid decomposition
3. Acts as a reservoir of nutrients
4. Forms very rapidly
48. Sickle cell anemia is caused by:
| 1. |
Chromosomal aberration |
2. |
Point mutation |
| 3. |
Non-disjunction event |
4. |
An arthropod vector |
49. Consider the given two statements:
| Assertion (A): |
Of the 46 chromosomes in a normal diploid human cell, half are maternally derived and half are paternally derived. |
| Reason (R): |
Sexual reproduction involves the fusion of two haploid gametes to produce a zygote and a new organism, in which every cell has two sets of chromosomes. |
| 1. |
Both (A) and (R) are True and (R) correctly explains the (A). |
| 2. |
Both (A) and (R) are True but (R) does not correctly explain the (A). |
| 3. |
(A) is True but (R) is False. |
| 4. |
Both (A) and (R) are False. |
50. Monascus purpureus is the yeast used commercially in the production of:
| 1. |
Ethanol |
| 2. |
Streptokinase for removing clots from the blood vessels |
| 3. |
Citric acid |
| 4. |
Blood cholesterol-lowering statins |
51. Male gametophyte in angiosperms produces?
1. Two sperms and a vegetative cell
2. Single sperm and a vegetative cell
3. Single sperm and two vegetative cells
4. Three sperms
52. Consider the given two statements:
| Assertion (A): |
Inbreeding increases the chances of offspring being affected by recessive traits. |
| Reason (R): |
Inbreeding increases the proportion of homozygous individuals in a population. |
| 1. |
Both (A) and (R) are True and (R) correctly explains the (A). |
| 2. |
Both (A) and (R) are True but (R) does not correctly explain the (A). |
| 3. |
(A) is True but (R) is False. |
| 4. |
Both (A) and (R) are False. |
53. In the mitochondrial electron transport chain, Complex IV refers to:
1. NADH dehydrogenase
2. Cytochrome c oxidase complex
3. Ubiquinone
4. ATP synthase
54. Deficiency of which of the given groups of elements causes necrosis?
1. Ca, Mg, Cu
2. N, S, Fe
3. N, S, Mo
4. K, S, Mo
Biology-I-Section-B
55. The taxonomic unit ‘Phylum’ in the classification of animals is equivalent to which hierarchical level in classification of plants?
| 1. |
Class |
2. |
Order |
| 3. |
Division |
4. |
Family |
56. Study the Assertion statement followed by the Reason statement given below:
| Assertion (A): |
Mosses reduce the impact of falling rain and prevent soil erosion. |
| Reason (R): |
Mosses form dense mats on the soil. |
| 1. |
(A) is True and (R) is False. |
| 2. |
Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 3. |
Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 4. |
(A) is False and (R) is True. |
57. In the dicot root, the vascular cambium originates from:
| 1. |
Tissue located below the phloem bundles and a portion of pericycle tissue above protoxylem |
| 2. |
Cortical region |
| 3. |
Parenchyma between endodermis and pericycle |
| 4. |
Intrafascicular and interfascicular tissue in a ring |
58. Amyloplasts, leucoplasts and aleuroplasts are:
| 1. |
Chromoplasts containing accessory photosynthetic pigments |
| 2. |
Pro-plastids capable of developing into any types of plastids |
| 3. |
Plastids found in green algae, photosynthesizing bacteria and protists |
| 4. |
Types of leucoplasts that store nutrients |
59. Morels and truffles:
| 1. |
are pathogenic fungi causing rust and smut |
| 2. |
do not reproduce sexually |
| 3. |
do not produce asexual spores |
| 4. |
are delicacies belonging to ascomycetes |
60. Floral formula of Solanum nigrum represents
1. Epitepalous condition
2. Epipetalous androecium
3. Polyserpalous calyx
4. Tricarpellary gynoecium
Biology-II-Section-A
61. During the concentration of urine by human kidneys:
| I: |
Small amounts of urea is transported back to medullary interstitium by ascending portion of vasa recta |
| II: |
NaCl is returned to the medullary interstitium by the descending portion of vasa recta |
1. Only
I is correct
2. Only
II is correct
3. Both
I and
II are correct
4. Both
I and
II are incorrect
62. Which of the following statements are correct with respect to vital capacity?
| (a) |
It includes ERV, TV and IRV |
| (b) |
Total volume of air a person can inspire after a normal expiration |
| (c) |
The maximum volume of air a person can breathe in after forced expiration |
| (d) |
It includes ERV, RV and IRV. |
| (e) |
The maximum volume of air a person can breathe out after a forced inspiration. |
Choose the most appropriate answer from the options given below:
| 1. |
(b), (d) and (e) |
2. |
(a), (c) and (d) |
| 3. |
(a), (c) and (e) |
4. |
(a) and (e) |
63. Consider the two statements:
| Statement I: |
When swallowing, the soft palate would have to move downward in order to prevent food from entering the lungs. |
| Statement II: |
When swallowing the epiglottis allows the food to enter the larynx. |
1. Statement I is correct: Statement II is incorrect
2. Statement I is incorrect: Statement II is correct
3. Statement I is correct: Statement II is correct
4. Statement I is incorrect: Statement II is incorrect
64. Using recombinant DNA technology, genes from a donor cell can be implanted into a bacterium for DNA replication and protein synthesis. The kind of cell(s) that can be used as gene donors in this technology is/are:
1. Bacteria only
2. Either yeast or bacteria only
3. Eukaryotic cells only
4. Any kind of cell
65. Which alphabet represent the ampulla in the given figure?

1. A
2. B
3. C
4.D
66. Name the ion responsible for unmasking of active sites for myosin for cross-bridge activity during muscle contraction:
| 1. |
Calcium |
2. |
Magnesium |
| 3. |
Sodium |
4. |
Potassium |
67. If we represent the chemical composition of living tissue from abundance point of view, the most abundant chemical in living organisms is:
| 1. |
Water |
2. |
Carbohydrates |
| 3. |
Proteins |
4. |
Lipids |
68. ART stands for:
1. Anti Retroviral Transcription
2. Alternate Reproductive Technology
3. Assisted Reproductive Technology
4. Anti Retroviral Translation
69. Figure shows blood circulation in humans with labels A to D. Select the option which gives correct identification of label and functions of the part:

| 1. |
B - Capillary - thin without muscle layers and wall two-cell layer thick |
| 2. |
C - Vein - thin-walled and blood flows in jerks/spurts |
| 3. |
D - Pulmonary vein - takes oxygenated blood to heart PO2=95 mm Hg |
| 4. |
A - Artery- thick-walled and blood flows evenly |
70. Over 95 % of all transgenic animals are:
1. Mice
2. Pigs
3. Sheep
4. Rabbits
71.
| Assertion(A): |
Smoking can raise blood pressure and increase heart rate. |
| Reason(R): |
Nicotine stimulates adrenal glands to release adrenaline and noradrenaline into the blood circulation, both of which raise blood pressure and increase heart rate |
| 1. |
Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. |
Both (A) and (R) are true and (R) is not the correct explanation of (A). |
| 3. |
(A) is true but (R) is false. |
| 4. |
(A) is false but (R) is true. |
72. Which part of our brain is involved in thermoregulation and osmoregulation?
1. Corpora quadrigemina
2. Cerebellum
3. Hypothalamus
4. Pons varolii
73. When compared to the vertebrates, invertebrates:
| 1. |
do not have any endocrine system. |
| 2. |
possess very simple endocrine systems with few hormones. |
| 3. |
possess very complex endocrine systems with a large number of hormones. |
| 4. |
produce only steroidal hormones. |
74. Which of the following statement is correct?
| 1. |
Mutations are random and directional |
| 2. |
Darwinian variations are small and non-directional |
| 3. |
According to Darwin, any population has built in variation in characteristics |
| 4. |
Gene flow occurs amongst the popultaions of different species |
Biology-II-Section-B
75. The single ventricle in the heart of a frog:
| 1. |
opens into a sac-like conus arteriosus on the dorsal side of the heart. |
| 2. |
opens into a sac-like conus arteriosus on the ventral side of the heart. |
| 3. |
opens into a dilated chamber sinus venosus on the ventral side of the heart. |
| 4. |
opens into a dilated chamber sinus venosus on the dorsal side of the heart. |
76. The heart, trachea, esophagus and associated structures are located in the space between the two lungs called as:
1. mediastinum
2. pleural cavity
3. thoracic cavity
4. parietal cavity
77. Goblet cells, present in alimentary canal, are used in:
1. Absorption
2. Protection
3. Secretion of mucus
4. Contraction
78. Given below are two statements: one is labelled as Assertion (A) and the other is labelled as Reason (R):
| Assertion (A): |
Rh incompatibility related complication does not arise in first pregnancy even if an Rh negative mother is carrying an Rh positive foetus. |
| Reason (R): |
Rh antigens are proteins. |
In the light of the above statements choose the correct answer from the options given below:
| 1. |
Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. |
Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. |
(A) is True but (R) is False. |
| 4. |
Both (A) and (R) are False. |
79. In the DNA double helix:
| I: |
At each step of ascent, the strand turns 34º. |
| II: |
The rise per base pair would be 0.36 nm. |
| 1. |
Only I is correct |
| 2. |
Only II is correct |
| 3. |
Both I and II are correct |
| 4. |
Both I and II are incorrect |
80. All the following animals are Urochordates except:
1. Ascidia
2. Salpa
3. Doliolum
4. Branchiostoma
*If above link doesn't work, please go to test link from where you got the pdf and fill OMR from there
CLICK HERE to get FREE ACCESS for 2 days of ANY NEETprep course