A cylindrical tube of uniform cross-sectional area A is fitted with two air tight frictionless pistons. The pistons are connected to each other by a metallic wire. Initially, the pressure of the gas is and temperature is . Atmospheric pressure is also . Now the temperature of the gas is increased to 2 . The tension in the wire will be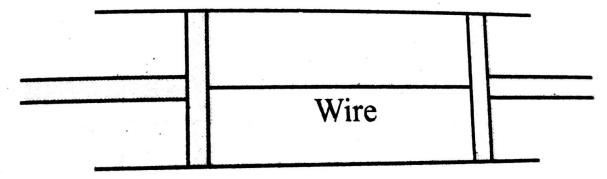
(1) 2A
(2) A
(3) A/2
(4) 4A
Which of the lines in figure, best reflects (on the log-log scale) the temperature dependence of the root mean square velocity of a molecule?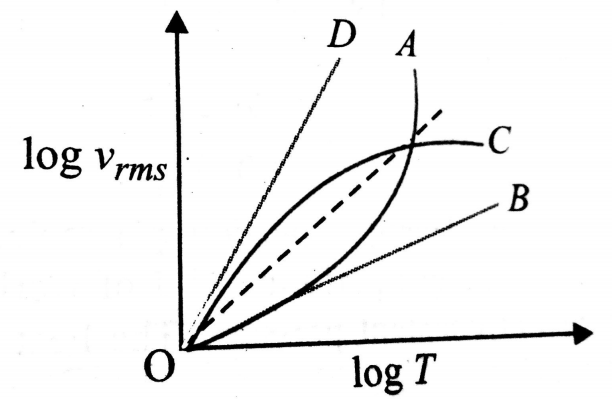
1. D
2. A
3. C
4. B
In the following V-T diagram, what is the relation between 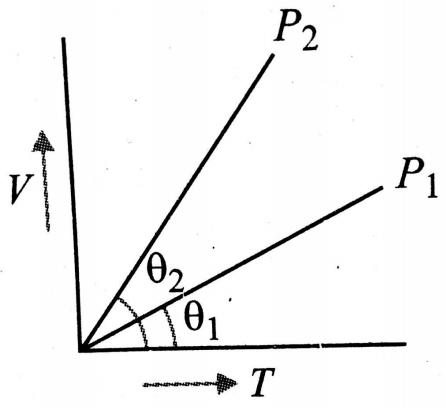
(1)
(2)
(3)
(4) Cannot be predicted
PV versus T graph of equal masses of , He and is shown in fig. 12.44. Choose the correct alternative.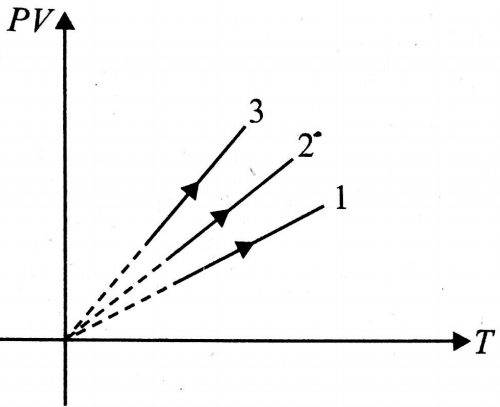
(1) 1 corresponds to , 2 to He and 3 to
(2) 1 corresponds to He, 2 to and 3 to
(3) 1 corresponds to , 2 to and 3 to He
(4) 1 corresponds to , 2 to He and 3 to
When an ideal diatomic gas is heated at constant pressure, the fraction of the heat energy supplied which increase the internal energy of the gas is
(1) 2/5
(2) 3/5
(3) 3/7
(4) 5/7
The average kinetic energy of molecule of a gas sample varies as K.E.= . This sample contains
(1) Monatomic gas
(2) Mixture of monatomic and diatomic gas
(3) Mixture of monatomic and polyatomic gas
(4) Both (2) and (3) are possible
An ideal monatomic gas is taken through a process in which . The molar heat capacity of the gas for the process is
(1) R
(2) 2R
(3) 3R
(4) 4R
A monatomic ideal gas, initially at temperature is enclosed in a cylinder fitted with a frinctionless piston. The gas is allowed to expand adibatically to a temperature by releasing the piston suddenly. If are the lengths of the gas column before and after expansion repectively, then is given by
(1)
(2)
(3)
(4)
Four mole of hydrogen, two moles of helium and one mole of water vapour form an ideal gas mixture. What is the molar specific heat at constant pressure of mixture?
(1)
(2)
(3) R
(4)
Two containers of equal volumes contain the same gas at the same pressure at temperature kelvin. On joining the vessels, the gas reaches a common temperature T which equals
(1)
(2)
(3)
(4)
The equation of the state of a gas is given by PV=nRT+Pb. If 2 moles of a gas is isothermally expanded from volume V to 2 V, the work done during the process is
(1)
(2) 2RT ln
(3) RT ln
(4) 2RT ln
An ideal gas is taken around the cycle ABCA as shown in P-V diagram. The net workdone by the gas during the cycle is equal to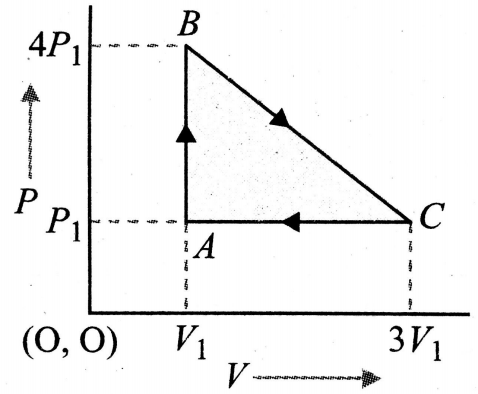
(1) 12
(2) 6
(3) 3
(4)
An ideal gas of mass m in state A goes to another state B via three different process as shown in fig. 12.51. If denote the heat absorbed by the gas along the three paths, then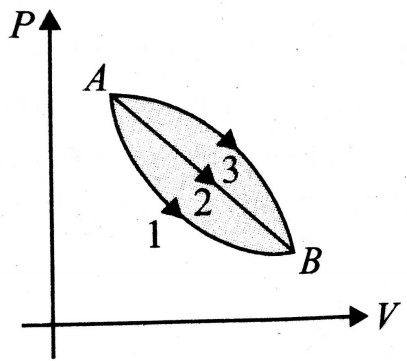
(1)
(2)
(3)
(4)
In a cyclic process shown in the fig. 12.53, an ideal gas is adiabatically taken from B to A. The work done on the gas during the process is 30 J. When the gas is taken from A to B, the heat absorbed by the gas is 20 J. The change in internal energy of the gas in the process A to B is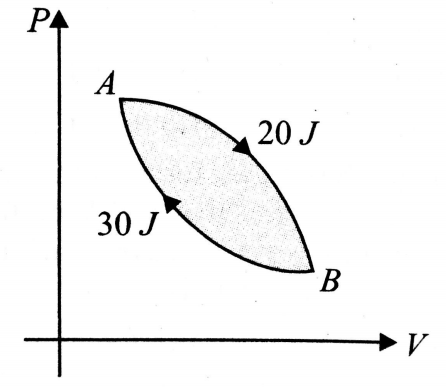
(1) 20 J
(2) -30 J
(3) 50 J
(4) -10 J
If a sample of a gas follows process AB, then which of the following is incorrect about AB?
(1)
(2) W>0
(3) Q=W
(4)
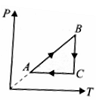
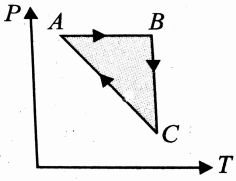
A thermodynamic cycle on an ideal gas is shown in the V-T graph. Which of the following P-T graphs represents the same cycle.
(1) 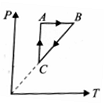 (2)
(2) 
(3)  (4)
(4) 
A Cyclic process contains four steps AB, BC, CD and DA. Heat involved in different process is given as
Then efficiency of process is
(1) 5/8
(2) 3/8
(3) 1/4
(4) 1/2
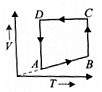
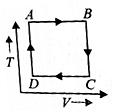
A cyclic process ABCD is shown in the P-V diagram. Which of the following curves represent the same process? (BC and DA are isothermal process)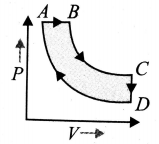
(1) 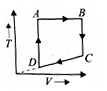 (2)
(2) 
(3) 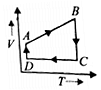 (4)
(4) 
The efficiency of a Carnot cycle is 1/6. If on reducing the temperature of sink by 65, the efficiency becomes 1/3. The initial and final temperatures between which the cycle is working are
(1) 117, 52
(2) 217, 52
(3) 317, 52
(4) 17, 52
A uniform tube closed at one end, contains a pallet of mercury 4 cm long. When the tube is kept vertical with closed and upward, the length of air column between closed end and mercury pallet is 10 cm. When the tube is inverted so that open end is upwards, find the length of trapped air column (in cm) trapped. Take atmospheric pressure equal to 76 cm of Hg.
(1) 9 cm
(2) 10 cm
(3) 8 cm
(4) None