Among the following species, the one that has the highest bond energy is:
1.
2.
3.
4.
When an electron jumps from n=5 to n=1 in a hydrogen atom, the number of spectral lines obtained is
| 1. | 3 | 2. | 4 |
| 3. | 6 | 4. | 10 |
When a solution of acetic acid was titrated with NaOH, the pH of the solution when half of the acid, neutralised was 4.2. Dissociation constant of the acid is
1. 6.31 × 10-5
2. 3.2 × 10-5
3. 8.7 × 10-8
4. 6.42 × 10-4
In the conversion, H2SO4 H2S2O8 which process occurs?
1. Oxidation
2. Reduction
3. Oxidation as well as reduction
4. Neither oxidation nor reduction
One mole of an ideal gas is taken from state A to state B by three different processes, (A) ACB (B) ADB (C) AEB as shown in the P-V diagram. The heat absorbed by the gas is
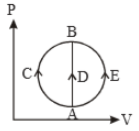
1. greater in process (B) than in (A)
2. the least in process (B)
3. the same in (A) and (C)
4. less in (C) then in (B)