This video is available for NEETprep Video Course students only
You’ve reached the end of your free Videos limit.
#35 | Application of First Law to Isochoric & Cyclic Processes
(Physics) > Thermodynamics
My Notes
Create your notes while watching video by clicking on icon in video player.
Related Practice Questions :
A system is taken from state \(A\) to state \(B\) along two different paths \(1\) and \(2.\) If the heat absorbed and work done by the system along these two paths are \(Q_1,Q_2\) and \(W_1,W_2\) respectively, then
1. \(Q_1=Q_2\)
2. \(W_1=W_2\)
3. \(Q_1-W_1=Q_2-W_2\)
4. \(Q_1+W_1=Q_2+W_2\)
65%
Level 2: 60%+
Hints
Unlock IMPORTANT QUESTION
This question was bookmarked by 5 NEET 2025 toppers during their NEETprep journey. Get Target Batch to see this question.
✨ Perfect for quick revision & accuracy boost
Buy Target Batch
Access all premium questions instantly
Unlock IMPORTANT QUESTION
This question was bookmarked by 5 NEET 2025 toppers during their NEETprep journey. Get Target Batch to see this question.
✨ Perfect for quick revision & accuracy boost
Buy Target Batch
Access all premium questions instantly
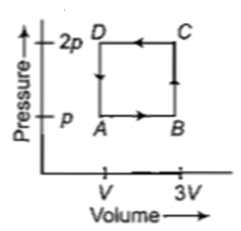
Figure below shows two paths that may be taken by a gas to go from a state \(A\) to a state \(C.\) In process \(AB,\) \(400\text{ J}\) of heat is added to the system and in process \(BC,\) \(100\text{ J}\) of heat is added to the system. The heat absorbed by the system in the process \(AC\) will be-
1. \(380\text{ J}\)
2. \(500\text{ J}\)
3. \(460\text{ J}\)
4. \(300\text{ J}\)
58%
Level 3: 35%-60%
NEET - 2015
Hints