Thermodynamics illustrates why only a certain reducing element and a minimum specific temperature are suitable for reduction of a metal oxide to the metal in an extraction.
After studying this Unit, you will be able to:
A few elements like carbon, sulphur, gold and noble gases, occur in free state while others in combined forms in the earth’s crust. The extraction and isolation of an element from its combined form involves various principles of chemistry. A particular element may occur in a variety of compounds. The process of metallurgy and isolation should be such that it is chemically feasible and commercially viable. Still, some general principles are common to all the extraction processes of metals. For obtaining a particular metal, first we look for minerals which are naturally occurring chemical substances in the earth’s crust obtainable by mining.
Out of many minerals in which a metal may be found, only a few are viable to be used as sources of that metal. Such minerals are known as ores. Rarely, an ore contains only a desired substance. It is usually contaminated with earthly or undesired materials known as gangue. The extraction and isolation of metals from ores involve the following major steps:
• Concentration of the ore,
• Isolation of the metal from its concentrated ore, and
• Purification of the metal.
The entire scientific and technological process used for isolation of the metal from its ores is known as metallurgy.
In the present Unit, first we shall describe various steps for effective concentration of ores. After that we shall discuss the principles of some of the common metallurgical processes. Those principles shall include the thermodynamic and electrochemical aspects involved in the effective reduction of the concentrated ore to the metal.
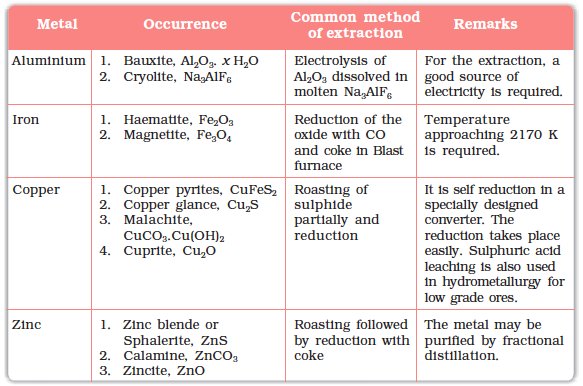
Elements vary in abundance. Among metals, aluminium is the most abundant. It is the third most abundant element in earth’s crust (8.3% approx. by weight). It is a major component of many igneous minerals including mica and clays. Many gemstones are impure forms of Al2O3 and the impurities range from Cr (in ‘ruby’) to Co (in ‘sapphire’). Iron is the second most abundant metal in the earth’s crust. It forms a variety of compounds and their various uses make it a very important element. It is one of the essential elements in biological systems as well
The principal ores of aluminium, iron, copper and zinc are given in Table 6.1.
Table 6.1: Principal Ores of Some Important Metals
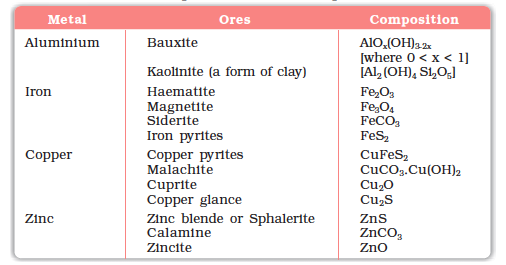
For the purpose of extraction, bauxite is chosen for aluminium. For iron, usually the oxide ores which are abundant and do not produce polluting gases (like SO2 that is produced in case iron pyrites) are taken. For copper and zinc, any of the listed ores (Table 6.1) may be used depending upon availability and other relevant factors. Before proceeding for concentration, ores are graded and crushed to reasonable size.
Removal of the unwanted materials (e.g., sand, clays, etc.) from the ore is known as concentration, dressing or benefaction. Before proceeding for concentration, ores are graded and crushed to reasonable size. Concentration of ores involves several steps and selection of these steps depends upon the differences in physical properties of the compound of the metal present and that of the gangue. The type of the metal, the available facilities and the environmental factors are also taken into consideration. Some of the important procedures for concentration of ore are described below.
This is based on the difference between specific gravities of the ore and thegangue particles. It is therefore a type of gravity separation. In one such process, an upward stream of running water is used to wash the powdered ore. The lighter gangue particles are washed away and the heavier ore particles are left behind.
This is based on differences in magnetic properties of the ore components. If either the ore or the gangue is attracted towards magnetic field, then the separation is carried out by this method. For example iron ores are attracted towards magnet, hence, non–magnetic impurities can be separted from them using magnetic separation. The powdered ore is dropped over a conveyer belt which moves over a magnetic roller (Fig.6.1) Magnetic substance remains attracted towards the belt and falls close to it.
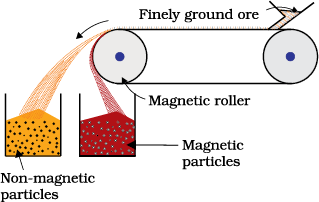
Fig. 6.1: Magnetic separation (schematic)
This method is used for removing gangue from sulphide ores. In this process, a suspension of the powdered ore is made with water. Collectors and froth stabilisers are added to it. Collectors (e.g., pine oils, fatty acids, xanthates, etc.) enhance non-wettability of the mineral particles and froth stabilisers (e.g., cresols, aniline) stabilise the froth.
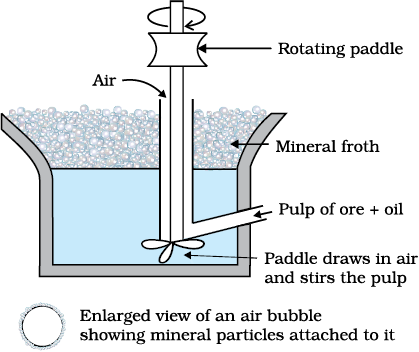
Fig. 6.2: Froth floatation process (schematic)
The mineral particles become wet by oils while the gangue particles by water. A rotating paddle agitates the mixture and draws air in it. As a result, froth is formed which carries the mineral particles. The froth is light and is skimmed off. It is then dried for recovery of the ore particles.
The Innovative Washerwoman
One can do wonders if he or she has a scientific temperament and is attentive to observations. A washerwoman had an innovative mind too. While washing a miner’s overalls, she noticed that sand and similar dirt fell to the bottom of the washtub. What was peculiar, the copper bearing compounds that had come to the clothes from the mines, were caught in the soapsuds and so they came to the top. One of her clients, Mrs. Carrie Everson was a chemist. The washerwoman told her experience to Mrs. Everson. The latter thought that the idea could be used for separating copper compounds from rocky and earth materials on large scale. This way an invention came up. At that time only those ores were used for extraction of copper, which contained large amounts of the metal. Invention of the Froth Floatation Method made copper mining profitable even from the low-grade ores. World production of copper soared and the metal became cheaper.
Leaching is often used if the ore is soluble in some suitable solvent. Following examples illustrate the procedure:
Bauxite is the principal ore of aluminium. It usually contains SiO2, iron oxides and titanium oxide (TiO2) as impurities. Concentration is carried out by heating the powdered ore with a concentrated solution of NaOH at 473 – 523 K and 35 – 36 bar pressure. This process is called digestion. This way, Al2O3 is extracted out as sodium aluminate. The impurity, SiO2 too dissolves forming sodium silicate. Other impurities are left behind.
Al2O3(s) + 2NaOH(aq) + 3H2O(l) → 2Na[Al(OH)4](aq) (6.1)
The sodium aluminate present in solution is neutralised by passing CO2 gas and hydrated Al2O3 is precipitated. At this stage, small amount of freshly prepared sample of hydrated Al2O3 is added to the solution. This is called seeding. It induces the precipitation.
2Na[Al(OH)4](aq) + CO2(g) → Al2O3.xH2O(s) + 2NaHCO3 (aq) (6.2)
Sodium silicate remains in the solution and hydrated alumina is filtered, dried and heated to give back pure Al2O3.
Al2O3.xH2O(s) Al2O3(s) + xH2O(g) (6.3)
Al2O3(s) + xH2O(g) (6.3)
(b) Other examples
In the metallurgy of silver and gold, the respective metal is leached with a dilute solution of NaCN or KCN in the presence of air, which supplies O2. The metal is obtained later by replacement reaction.
4M(s) + 8CN–(aq)+ 2H2O(aq) + O2(g) → 4[M(CN)2]– (aq) + 4OH–(aq) (M= Ag or Au) (6.4)
2[M(CN)2]- (aq) + Zn (s) → [Zn(CN)4]2- (aq) +2M (s) (6.5)
Intext Questions
6.1 Which of the ores mentioned in Table 6.1 can be concentrated by magnetic separation method?
6.2 What is the significance of leaching in the extraction of aluminium?
The concentrated ore must be converted into a form which is suitable for reduction. Usually the sulphide ore is converted to oxide before reduction because oxides are easier to reduce. Thus isolation of metals from concentrated ore involves two major steps viz.,
(a) conversion to oxide, and
(b) reduction of the oxide to metal.
(a) Conversion to oxide
Fe2O3.xH2O(s) 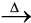 Fe2O3 (s) + xH2O(g) (6.6)
Fe2O3 (s) + xH2O(g) (6.6)
ZnCO3 (s)  ZnO(s) + CO2(g) (6.7)
ZnO(s) + CO2(g) (6.7)
CaCO3.MgCO3(s)  CaO(s) + MgO(s ) + 2CO2(g) (6.8)
CaO(s) + MgO(s ) + 2CO2(g) (6.8)
(ii) Roasting: In roasting, the ore is heated in a regular supply of air in a furnace at a temperature below the melting point of the metal. Some of the reactions involving sulphide ores are:
2ZnS + 3O2 → 2ZnO + 2SO2 (6.9)
2PbS + 3O2 → 2PbO + 2SO2 (6.10)
2Cu2S + 3O2 → 2Cu2O + 2SO2 (6.11)
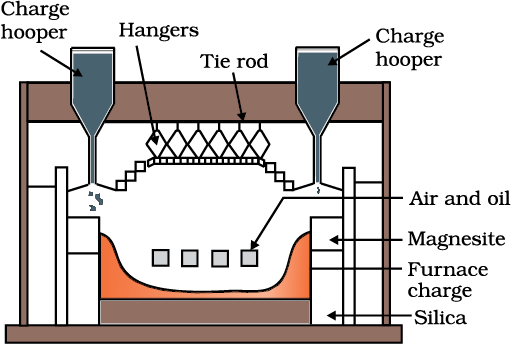
Fig. 6.3: A section of a modern reverberatory furnace
The sulphide ores of copper are heated in reverberatory furnace [Fig. 6.3]. If the ore contains iron, it is mixed with silica before heating. Iron oxide ‘slags of ’* as iron silicate and copper is produced in the form of copper matte which contains Cu2S and FeS.
FeO + SiO2 → FeSiO3 (slag) (6.12)
The SO2 produced is utilised for manufacturing H2SO4 .
* During metallurgy, ‘flux’ is added which combines with ‘gangue’ to form ‘slag’. Slag separates more easily from the ore than the gangue. This way, removal of gangue becomes easier.
(b) Reduction of oxide to the metal
Some basic concepts of thermodynamics help us in understanding the theory of metallurgical transformations. Gibbs energy is the most significant term. To understand the variation in the temperature required for thermal reductions and to predict which element will suit as the reducing agent for a given metal oxide (MxOy), Gibbs energy interpretations are made. The criterion for the feasibility of a thermal reduction is that at a given temperture Gibbs energy change of the reaction must be negative. The change in Gibbs energy, ∆G for any process at any specified temperature, is described by the equation:
∆G = ∆H – T∆S (6.14)
where, ∆H is the enthalpy change and ∆S is the entropy change for the process.For any reaction, this change could also be explained through the equation:
(6.15)
where, K is the equilibrium constant of the ‘reactant – product’ system at the temperature, T. A negative ΔG implies a +ve K in equation 6.15. And this can happen only when reaction proceeds towards products. From these facts we can make the following conclusions:
1. When the value of ∆G is negative in equation 6.14, only then the reaction will proceed. If ΔS is positive, on increasing the temperature (T), the value of TΔS would increase (ΔH < TΔS) and then ΔG will become –ve
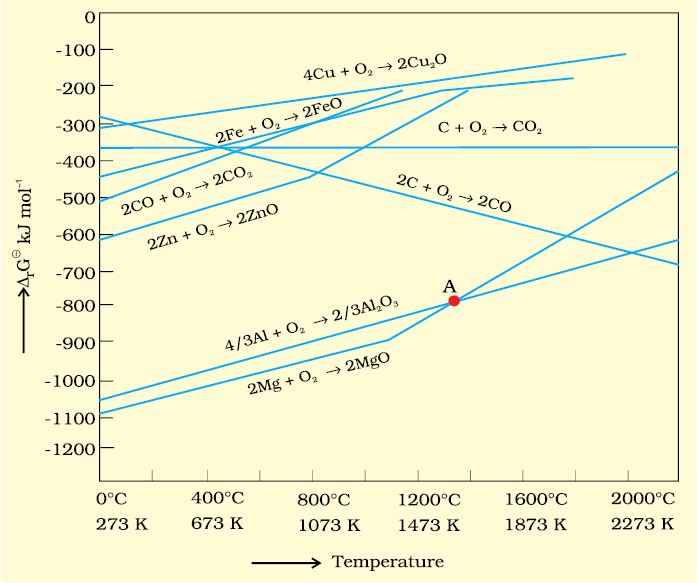
2. If reactants and products of two reactions are put together in a system and the net ΔG of the two possible reactions is –ve, the overall reaction will occur. So the process of interpretation involves coupling of the two reactions, getting the sum of their ΔG and looking for its magnitude and sign. Such coupling is easily understood through Gibbs energy (Δ) vs T plots for formation of the oxides (Fig. 6.4)
Ellingham Diagram
The graphical representation of Gibbs energy was first used by H.J.T.Ellingham. This provides a sound basis for considering the choice of reducing agent in the reduction of oxides. This is known as Ellingham Diagram. Such diagrams help us in predicting the feasibility of thermal reduction of an ore. The criterion of feasibility is that at a given temperature, Gibbs energy of the reaction must be negative.
(a) Ellingham diagram normally consists of plots of vs T for formation of oxides
of elements i.e., for the reaction,
2xM(s) + O2(g) → 2MxO(s)
In this reaction, the gaseous amount (hence molecular randomness) is decreasing from left to right due to the consumption of gases leading to a –ve value of ΔS which changes the sign of the second term in equation (6.14). Subsequently ΔG shifts towards higher side despite rising T (normally, ΔG decreases i.e., goes to lower side with increasing temperature). The result is +ve slope in the curve for most of the reactions shown above for formation of MxO(s).
(b) Each plot is a straight line except when some change in phase (s→liq or liq→g)
takes place. The temperature at which such change occurs, is indicated by an
increase in the slope on +ve side (e.g., in the Zn, ZnO plot, the melting is indicated
by an abrupt change in the curve).
(c) There is a point in a curve below which ΔG is negative (So MxO is stable). Above
this point, MxO will decompose on its own.
(d) In an Ellingham diagram, the plots of for oxidation (and therefore reduction
of the corresponding species) of common metals and some reducing agents are given. The values of , etc.(for formation of oxides) at different temperatures are depicted which make the interpretation easy.
(e) Similar diagrams are also constructed for sulfides and halides and it becomes clear why reductions of MxS is difficult. There, the of MxS is not compensated
Limitations of Ellingham Diagram
2. The interpretation of ∆rGƟ is based on K (∆GƟ = – RT lnK). Thus it is presumed that the reactants and products are in equilibrium:
MxO + Ared l xM + AredO
Suggest a condition under which magnesium could reduce alumina.
Solution
The two equations are:
(a) 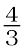 Al + O2 →
Al + O2 → 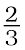 Al2O3 (b) 2Mg +O2 → 2MgO
Al2O3 (b) 2Mg +O2 → 2MgO
At the point of intersection of the Al2O3 and MgO curves (marked “A” in diagram 6.4), the ∆rG0 becomes ZERO for the reaction:
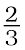 Al2O3 +2Mg → 2MgO +
Al2O3 +2Mg → 2MgO +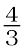 Al
Al
Below that point magnesium can reduce alumina.
Example 6.2
Although thermodynamically feasible, in practice, magnesium metal is not used for the reduction of alumina in the metallurgy of aluminium. Why ?
Solution
Temperatures below the point of intersection of Al2O3 and MgO curves, magnesium can reduce alumina. But the process will be uneconomical.
Example 6.3
Why is the reduction of a metal oxide easier if the metal formed is in liquid state at the temperature of reduction?
Solution
The entropy is higher if the metal is in liquid state than when it is in solid state. The value of entropy change (∆S) of the reduction process is more on positive side when the metal formed is in liquid state and the metal oxide being reduced is in solid state. Thus the value of ∆rGƟ becomes more on negative side and the reduction becomes easier.
(a) Extraction of iron from its oxides
At 500 – 800 K (lower temperature range in the blast furnace),
3 Fe2O3 + CO → 2 Fe3O4 + CO2 (6.28)
Fe3O4 + 4 CO → 3Fe + 4 CO2 (6.29)
Fe2O3 + CO → 2FeO + CO2 (6.30)
At 900 – 1500 K (higher temperature range in the blast furnace):
C + CO2 → 2 CO (6.31)
FeO + CO → Fe + CO2 (6.32)
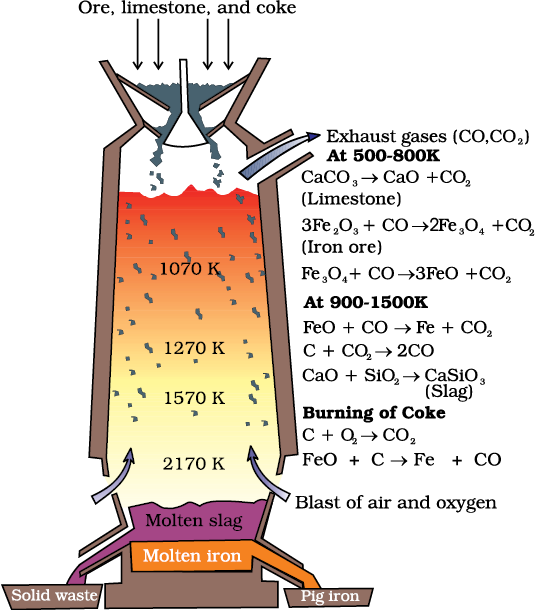
Fig. 6.5: Blast furnace
Limestone is also decomposed to CaO which removes silicate impurity of the ore as slag. The slag is in molten state and separates out from iron.
The iron obtained from Blast furnace contains about 4% carbon and many impurities in smaller amount (e.g., S, P, Si, Mn). This is known as pig iron and cast into variety of shapes. Cast iron is different from pig iron and is made by melting pig iron with scrap iron and coke using hot air blast. It has slightly lower carbon content (about 3%) and is extremely hard and brittle.
Further Reductions
Wrought iron or malleable iron is the purest form of commercial iron and is prepared from cast iron by oxidising impurities in a reverberatory furnace lined with haematite. The haematite oxidises carbon to carbon monoxide:
Fe2O3 + 3 C → 2 Fe + 3 CO (6.31)
Limestone is added as a flux and sulphur, silicon and phosphorus are oxidised and passed into the slag. The metal is removed and freed from the slag by passing through rollers.
(b) Extraction of copper from cuprous oxide [copper(I) oxide]
2Cu2S + 3O2 → 2Cu2O + 2SO2 (6.32)
The oxide can then be easily reduced to metallic copper using coke:
Cu2O + C → 2 Cu + CO (6.33)
In actual process, the ore is heated in a reverberatory furnace after mixing with silica. In the furnace, iron oxide ‘slags of’ as iron slicate is formed. Copper is produced in the form of copper matte. This contains Cu2S and FeS.
FeO + SiO2 → FeSiO3 (Slag) (6.34)
Copper matte is then charged into silica lined convertor. Some silica is also added and hot air blast is blown to convert the remaining FeS, FeO and Cu2S/Cu2O to the metallic copper. Following reactions take place:
2FeS + 3O2 → 2FeO + 2SO2 (6.35)
FeO + SiO2 → FeSiO3 (6.36)
2Cu2S + 3O2 → 2Cu2O + 2SO2 (6.37)
2Cu2O + Cu2S → 6Cu + SO2 (6.38)
The solidified copper obtained has blistered appearance due to the evolution of SO2 and so it is called blister copper.
(c) Extraction of zinc from zinc oxide
The reduction of zinc oxide is done using coke. The temperature in this case is higher than that in the case of copper. For the purpose of heating, the oxide is made into brickettes with coke and clay.
ZnO + C 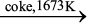 Zn + CO (6.39)
Zn + CO (6.39)
The metal is distilled off and collected by rapid chilling.
Intext Questions
6.3 The reaction,
Cr2O3+2Al → Al2O3+2Cr (∆GƟ= – 421kJ)
6.4 Is it true that under certain conditions, Mg can reduce Al2O3 and Al can reduce MgO? What are those conditions?
We have seen how principles of thermodyamics are applied to pyrometallurgy. Similar principles are effective in the reductions of metal ions in solution or molten state. Here they are reduced by electrolysis or by adding some reducing element.
In the reduction of a molten metal salt, electrolysis is done. Such methods are based on electrochemical principles which could be understood through the equation,
∆GƟ = – nEƟF (6.40)
Cu2+ (aq) + Fe(s) → Cu(s) + Fe2+ (aq) (6.41)
In simple electrolysis, the Mn+ ions are discharged at negative electrodes (cathodes) and deposited there. Precautions are taken considering the reactivity of the metal produced and suitable materials are used as electrodes. Sometimes a flux is added for making the molten mass more conducting.
Aluminium
In the metallurgy of aluminium, purified Al2O3 is mixed with Na3AlF6 or CaF2 which lowers the melting point of the mixture and brings conductivity. The fused matrix is electrolysed. Steel vessel with lining of carbon acts as cathode and graphite anode is used. The overall reaction may be written as:
2Al2O3 + 3C → 4Al + 3CO2 (6.42)
This process of electrolysis is widely known as Hall-Heroult process.
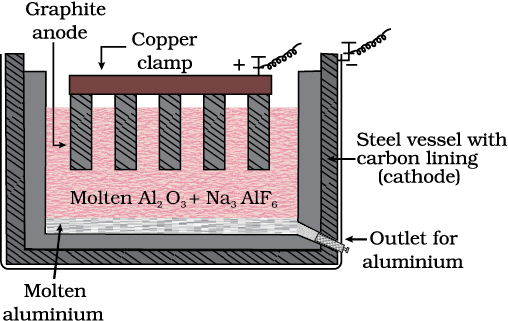
Fig. 6.7: Electrolytic cell for the extraction of aluminium
Thus electrolysis of the molten mass is carried out in an electrolytic cell using carbon electrodes. The oxygen liberated at anode reacts with the carbon of anode producing CO and CO2. This way for each kg of aluminium produced, about 0.5 kg of carbon anode is burnt away. The electrolytic reactions are:
Cathode: Al3+ (melt) + 3e– → Al(l) (6.43)
Anode: C(s) + O2– (melt) → CO(g) + 2e– (6.44)
C(s) + 2O2– (melt) → CO2 (g) + 4e– (6.45)
Copper from Low Grade Ores and Scraps
Copper is extracted by hydrometallurgy from low grade ores. It is leached out using acid or bacteria. The solution containing Cu2+ is treated with scrap iron or H2 (equations 6.40; 6.46).
Cu2+(aq) + H2(g) → Cu(s) + 2H+ (aq) (6.46)
Example 6.4
At a site, low grade copper ores are available and zinc and iron scraps are also available. Which of the two scraps would be more suitable for reducing the leached copper ore and why?
Solution
Zinc being above iron in the electrochemical series (more reactive metal is zinc), the reduction will be faster in case zinc scraps are used. But zinc is costlier metal than iron so using iron scraps will be advisable and advantageous.
Besides reductions, some extractions are based on oxidation particularly for non-metals. A very common example of extraction based on oxidation is the extraction of chlorine from brine (chlorine is abundant in sea water as common salt) .
2Cl–(aq) + 2H2O(l) → 2OH–(aq) + H2(g) + Cl2(g) (6.47)
The ∆GƟ for this reaction is + 422 kJ. When it is converted to EƟ (using ∆GƟ = – nEƟF), we get EƟ = – 2.2 V. Naturally, it will require an external emf that is greater than 2.2 V. But the electrolysis requires an excess potential to overcome some other hindering reactions (Unit–3, Section 3.5.1). Thus, Cl2 is obtained by electrolysis giving out H2 and aqueous NaOH as by-products. Electrolysis of molten NaCl is also carried out. But in that case, Na metal is produced and not NaOH.
4Au(s) + 8CN–(aq) + 2H2O(aq) + O2(g) → 4[Au(CN)2]–(aq) + 4OH–(aq) (6.48)
2[Au(CN)2]–(aq) + Zn(s) → 2Au(s) + [Zn(CN)4]2– (aq) (6.49)
In this reaction zinc acts as a reducing agent.
A metal extracted by any method is usually contaminated with some impurity. For obtaining metals of high purity, several techniques are used depending upon the differences in properties of the metal and the impurity. Some of them are listed below.
(a) Distillation (b) Liquation
(c) Electrolysis (d) Zone refining
(e) Vapour phase refining (f) Chromatographic methods
These are described in detail here.
(a) Distillation
This is very useful for low boiling metals like zinc and mercury. The impure metal is evaporated to obtain the pure metal as distillate.
(b) Liquation
In this method a low melting metal like tin can be made to flow on a sloping surface. In this way it is separated from higher melting impurities.
(c) Electrolytic refining
In this method, the impure metal is made to act as anode. A strip of the same metal in pure form is used as cathode. They are put in a suitable electrolytic bath containing soluble salt of the same metal. The more basic metal remains in the solution and the less basic ones go to the anode mud. This process is also explained using the concept of electrode potential, over potential, and Gibbs energy which you have seen in previous sections. The reactions are:
Anode: M → Mn+ + ne–
Cathode: Mn+ + ne– → M (6.50)
Copper is refined using an electrolytic method. Anodes are of impure copper and pure copper strips are taken as cathode. The electrolyte is acidified solution of copper sulphate and the net result of electrolysis is the transfer of copper in pure form from the anode to the cathode:
Anode: Cu → Cu2+ + 2 e–
Cathode: Cu2+ + 2e– → Cu (6.51)
Impurities from the blister copper deposit as anode mud which contains antimony, selenium, tellurium, silver, gold and platinum; recovery of these elements may meet the cost of refining. Zinc may also be refined this way.
(d) Zone refining
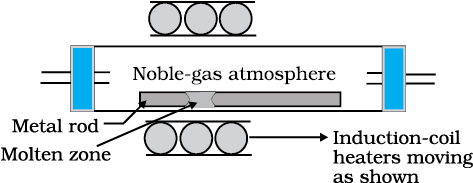
Fig. 6.8: Zone refining process
(e) Vapour phase refining
In this method, the metal is converted into its volatile compound which is collected and decomposed to give pure metal. So, the two requirements are:
(i) the metal should form a volatile compound with an available reagent,
(ii) the volatile compound should be easily decomposable, so that the recovery is easy.
Following examples will illustrate this technique.
Mond Process for Refining Nickel: In this process, nickel is heated in a stream of carbon monoxide forming a volatile complex named as nickel tetracarbonyl. This compex is decomposed at higher temperature to obtain pure metal.
Ni + 4CO  Ni(CO)4 (6.52)
Ni(CO)4 (6.52)
Ni(CO)4  Ni + 4CO (6.53)
Ni + 4CO (6.53)
van Arkel Method for Refining Zirconium or Titanium: This method is very useful for removing all the oxygen and nitrogen present in the form of impurity in certain metals like Zr and Ti. The crude metal is heated in an evacuated vessel with iodine. The metal iodide being more covalent, volatilises:
Zr + 2I2 → ZrI4 (6.54)
The metal iodide is decomposed on a tungsten filament, electrically heated to about 1800K. The pure metal deposits on the filament.
ZrI4 → Zr + 2I2 (6.55)
(f) Chromatographic methods
This method is based on the principle that different components of a mixture are differently adsorbed on an adsorbent. The mixture is put in a liquid or gaseous medium which is moved through the adsorbent.Different components are adsorbed at different levels on the column.
Later the adsorbed components are removed (eluted) by using suitable solvents (eluant). Depending upon the physical state of the moving medium and the adsorbent material and also on the process of passage of the moving medium, the chromatographic method* is given the name. In one such method the column of Al2O3 is prepared in a glass tube and the moving medium containing a solution of the components is in liquid form. This is an example of column chromatography. This is very useful for purification of the elements which are available in minute quantities and the impurities are not very different in chemical properties from the element to be purified. There are several chromatographic techniques such as paper chromatography, column chromatography, gas chromatography, etc. Procedures followed in column chromatography have been depicted in Fig. 6.8.
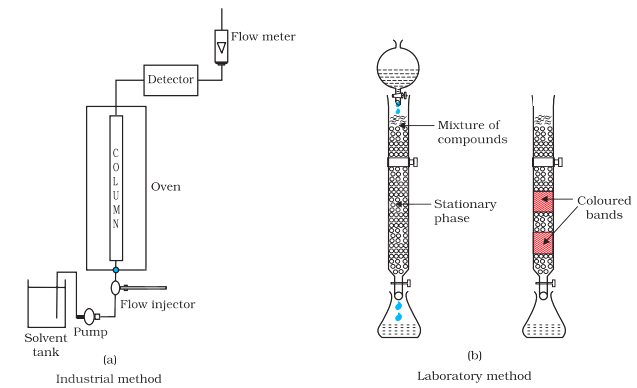
Fig. 6.8: Schematic diagrams showing column chromatography
Looking it the other way, chromatography in general, involves a mobile phase and a stationary phase. The sample or sample extract is dissolved in a mobile phase. The mobile phase may be a gas, a liquid or a supercritical fluid. The stationary phase is immobile and immiscible (like the Al2O3 column in the example of column chromatography above). The mobile phase is then forced through the stationary phase. The mobile phase and the stationary phase are chosen such that components of the sample have different solubilities in the two phases. A component which is quite soluble in the stationary phase takes longer
time to travel through it than a component which is not very soluble in the stationary phase but very soluble in the mobile phase. Thus sample components are separated from each other as they travel through the stationary phase. Depending upon the two phases and the way sample is inserted/injected, the chromatographic technique is named. These methods have been described in detail in Unit 12 of Class XI text book (12.8.5).
Aluminium foils are used as wrappers for food materials. The fine dust of the metal is used in paints and lacquers. Aluminium, being highly reactive, is also used in the extraction of chromium and manganese from their oxides. Wires of aluminium are used as electricity conductors. Alloys containing aluminium, being light, are very useful.
Copper is used for making wires used in electrical industry and for water and steam pipes. It is also used in several alloys that are rather tougher than the metal itself, e.g., brass (with zinc), bronze (with tin) and coinage alloy (with nickel).
Zinc is used for galvanising iron. It is also used in large quantities in batteries. It is constituent of many alloys, e.g., brass, (Cu 60%, Zn 40%) and german silver (Cu 25-30%, Zn 25-30%, Ni 40–50%). Zinc dust is used as a reducing agent in the manufacture of dye-stuffs, paints, etc.
Although modern metallurgy had exponential growth after Industrial Revolution, many modern concepts in metallurgy have their roots in ancient practices that predated the Industrial Revolution. For over 7000 years, India has had high tradition of metallurigical skills. Ancient Indian metallurgists have made major contributions which deserve their place in metallurgical history of the world. In the case of zinc and high–carbon steel, ancient India contributed significantly for the developemnt of base for the modern metallurgical advancements which induced metallurgical study leading to Industrial Revolution.
Metals are required for a variety of purposes. For this, we need their extraction from the minerals in which they are present and from which their extraction is commercially feasible.These minerals are known as ores. Ores of the metal are associated with many impurities. Removal of these impurities to certain extent is achieved in concentration steps. The concentrated ore is then treated chemically for obtaining the metal. Usually the metal compounds (e.g., oxides, sulphides) are reduced to the metal. The reducing agents used are carbon, CO or even some metals. In these reduction processes, the thermodynamic and electrochemical concepts are given due consideration. The metal oxide reacts with a reducing agent; the oxide is reduced to the metal and the reducing agent is oxidised. In the two reactions, the net Gibbs energy change is negative, which becomes more negative on raising the temperature. Conversion of the physical states from solid to liquid or to gas, and formation of gaseous states favours decrease in the Gibbs energy for the entire system. This concept is graphically displayed in plots of ∆GƟ vs T (Ellingham diagram) for such oxidation/reduction reactions at different temperatures. The concept of electrode potential is useful in the isolation of metals (e.g., Al, Ag, Au) where the sum of the two redox couples is positive so that the Gibbs energy change is negative. The metals obtained by usual methods still contain minor impurities. Getting pure metals requires refining. Refining process depends upon the differences in properties of the metal and the impurities. Extraction of aluminium is usually carried out from its bauxite ore by leaching it with NaOH. Sodium aluminate, thus formed, is separated and then neutralised to give back the hydrated oxide, which is then electrolysed using cryolite as a flux. Extraction of iron is done by reduction of its oxide ore in blast furnace. Copper is extracted by smelting and heating in a reverberatory furnace. Extraction of zinc from zinc oxides is done using coke. Several methods are employed in refining the metal. Metals, in general, are very widely used and have contributed significantly in the development of a variety of industries.
6.1 Copper can be extracted by hydrometallurgy but not zinc. Explain.
NEETprep Answer6.2 What is the role of depressant in froth floatation process?
NEETprep Answer6.3 Why is the extraction of copper from pyrites more difficult than that from its oxide ore through reduction?
NEETprep Answer6.4 Explain: (i) Zone refining (ii) Column chromatography.
NEETprep Answer6.5 Out of C and CO, which is a better reducing agent at 673 K ?
NEETprep Answer6.6 Name the common elements present in the anode mud in electrolytic refining of copper. Why are they so present ?
NEETprep Answer6.7 Write down the reactions taking place in different zones in the blast furnace during the extraction of iron.
NEETprep Answer6.8 Write chemical reactions taking place in the extraction of zinc from zinc blende.
NEETprep Answer6.9 State the role of silica in the metallurgy of copper.
NEETprep Answer6.10. “Chromatography”, What do you understand by this term?
NEETprep Answer6.11. What is the criterion followed while selecting the stationary phase of chromatography?
NEETprep Answer6.12 Describe a method for refining nickel.
NEETprep Answer6.13 How can you separate alumina from silica in a bauxite ore associated with silica? Give equations, if any.
NEETprep Answer6.14 Giving examples, differentiate between ‘roasting’ and ‘calcination’.
NEETprep Answer6.15 How is ‘cast iron’ different from ‘pig iron”?
NEETprep Answer6.16 Differentiate between “minerals” and “ores”.
NEETprep Answer6.17 Why copper matte is put in silica lined converter?
NEETprep Answer6.18 What is the role of cryolite in the metallurgy of aluminium?
NEETprep Answer6.19 How is leaching carried out in case of low grade copper ores?
NEETprep Answer6.20 Why is zinc not extracted from zinc oxide through reduction using CO?
NEETprep Answer6.21 The value of ∆fGƟ for formation of Cr2 O3 is – 540 kJmol−1and that of Al2 O3 is – 827 kJmol−1. Is the reduction of Cr2 O3 possible with Al ?
6.22 Out of C and CO, which is a better reducing agent for ZnO ?
NEETprep Answer6.23 The choice of a reducing agent in a particular case depends on thermodynamics factor. How far do you agree with this statement? Support your opinion with two examples.
NEETprep Answer6.24 Name the processes from which chlorine is obtained as a by-product. What will happen if an aqueous solution of NaCl is subjected to electrolysis?
6.25 What is the role of graphite rod in the electrometallurgy of aluminium?
NEETprep Answer6.26 Outline the principles of refining of metals by the following methods:
(i) Zone refining
(ii) Electrolytic refining
(iii) Vapour phase refining
NEETprep Answer6.27 Predict conditions under which Al might be expected to reduce MgO.
(Hint: See Intext question 6.4)
NEETprep Answer1. In the extraction of chlorine by electrolysis of brine ...........
(a) oxidation of ion to chlorine gas occurs
(b) reduction of ion to chlorine gas occurs
(c) for overall reaction has negative value
(d) a displacement reaction takes place
NEETprep Answer
2. When copper ore is mixed with silica, in a reverberatory furnace copper matte is produced. The copper matte contains
(a) sulphides of copper (l) and iron (ll)
(b) sulphides of copper (ll) and iron (lll)
(c) sulphides of copper(l) and iron (ll)
(d) sulphides of copper (l) and iron (lll)
NEETprep Answer
3. Which of the following reactions is an example of autoreduction?
NEETprep Answer
4. A number of elements are available in earth’s crust but most abundant elements are .........
(a) Al and Fe
(b) Al and Cu
(c) Fe and Cu
(d) Cu and Ag
NEETprep Answer
5. Zone refining is hased on the principle that ..........
(a) impurities of low boiling metals can be separated by distillation.
(b) impurities are more soluble in molten metal than in solid metal.
(c) different components of a mixture are differently adsorbed on an adsorbent.
(d) vapours of volatile compound can be decomposed in pure metal.
NEETprep Answer
6. In the extraction of copper from its sulphide ore, the metal is formed by the reduction of with
(a)
(b)
(c)
(d)
NEETprep Answer
7. Brine is electrolysed by using inert electrodes. The reaction at anode is .........
(a) ; = 1.36 V
(b) ; = 1.23 V
(c) ; = 2.71V
; = 0.00 V
NEETprep Answer
8. In the metallurgy of aluminium ............
(a) is oxidised to (s).
(b) graphide anode is oxidised to carbon monoxide and carbon dioxide.
(c) oxidation state of oxygen changesin the reaction at anode.
(d) oxidation state of oxygen changes in the overall reaction involved in the process.
NEETprep Answer9. Electrolytic refining is used to purify which of the following metals?
(a) Cu and Zn
(b) Ge and Si
(c) Zr and Ti
(d) Zn and Hg
NEETprep Answer10. Extraction of gold and silver involves leaching the metal with ion. The metal is recovered by ......
(a) displacement of metal by some other metal from the complex ion.
(b) roasting of metal complex.
(c) calcination followed byroasting.
(d) thermal decomposition of metal complex.
NEETprep Answer
Direction (Q. Nos. 11-13) Answer the questions on the basis of figure
11. Choose the correct option of temperature at which carbon reduces FeO to iron and produces CO.
(a) Below temperature at point A
(b) Approximately at the temperature corresponding to point A
(c) Above temperature at point A but below temperature at point D
(d) Above temperature at point A
NEETprep Answer12. Below point ‘A’ FeO can .............
(a) be reduced by carbon monoxide only.
(b) be reduced by both carbon monoxide and carbon.
(c) be reduced by carbon only.
(d) not be reduced by both carbon and carbon monoxide.
NEETprep Answer
13. For the reduction of FeO at the temperature corresponding to point D, which of the following statements is correct?
(a) value for the overall reduction reaction with carbon monoxideis zero.
(b) value for the overall reduction reaction with a mixture of 1 mol carbon and 1 mol oxygen is positive.
(c) value for the overall reduction reaction with a mixture of 2 mol carbon and 1 mol oxygen will be positive.
(d) value for the overail reduction reaction with carbon monoxideis negative.
NEETprep Answer
Multiple Choice Questions (More than One Options)
14. At the temperature corresponding to which of the points in Fig. FeO will be reduced to Fe by coupling the reaction with all of the following reactions?
(a) Point A
(b) Point B
(c) Point D
(d) Point E
NEETprep Answer
15. Which of the following options are correct?
(a) Cast iron is obtained by remelting pig iron with scrap iron and coke using hot air blast.
(b) In extraction ofsilver, silver is extracted as cationic complex.
(c) Nickel is purified by zone refining.2
(d) Zr and Ti are purified by van Arkel method.
NEETprep Answer
16. Tn the extraction of aluminium by Hall-Heroult process, purified is mixed with to
(a) lower the melting point of
(b) increase the conduclivily of molten mixture.
(c) reduce into
(d) acts as catalyst
NEETprep Answer
17. Which of the following statements is correct about the role of substances added in the froth floatation process?
(a) Collectors enhance the non-wettahility of the mineral particles.
(b) Collectors enhance the wettability of gangue particles,
(c) By using depressants in the process two sulphide ores can be separated.
(d) Froth stabilisers decrease wettability of gangue.
NEETprep Answer
18. In the froth floatation process, zinc sulphide and lead sulphide can be separated by ...............
(a) using collectors
(b) adjusting the proportion of oil to water
(c) using depressant
(d) using froth stabilisers
NEETprep Answer
19. Common impurities present in bauxite are ........
(a)
(b)
(c)
(d)
NEETprep Answer
20. Which of the following ores are concentrated by froth floatation?
(a) Haematite (b) Galena (c) Copper pyrites (d) Magnetite
NEETprep Answer
21. Which of the following reactions occur during calcination?
NEETprep Answer
22. For the metallurgical process of which of the ores calcined ore can be reduced by carbon?
(a) Haematite
(b) Calamine
(c) Iron pyrites
(d) Sphalerite
NEETprep Answer
23. The main reactions occurring in blast furnace during extraction of iron from haematite ore ..........
NEETprep Answer
24. In which of the following method of purification, metal is converted to its volatile compound which is decomposed to give pure metal?
(a) Heating with stream of carbon monoxide
(b) Heating with iodine
(c) Liquation
(d) Distillation
NEETprep Answer
25. Which of the following statements are correct?
(a) A depressant prevents certain type of particle to come to the froth.
(b) Copper matte contains and .
(c) The solidified copper obtained from reverberatory furnace has blistered appearance due to evolution of during the extraction.
(d) Zinc can be extracted by self-reduction.
NEETprep Answer
26. In the extraction of chlorine from brine ............
(a) for the overall reaction is negative.
(b) for the overall reaction is positive.
(c) for the overall reaction has negative value.
(d) for the overall reaction has positive value.
NEETprep Answer
27. Why is an external emf of more than 2.2V required for the extraction of from brine?
NEETprep Answer
28. At temperature above 1073 K, coke can be used to reduce FeO to Fe. How can you justify this reduction with Ellingham diagram?
NEETprep Answer
29. Wrought iron is the purest form of iron. Write a reaction used for the preparation of wrought iron from cast iron. How can the impurities of sulphur, silicon and phosphorus be removed from cast iron?
NEETprep Answer
30. How is copper extracted from low grade copper ores?
NEETprep Answer
31. Write two basic requirements for refining of a metal by Mond’s process and by van Arkel Method.
NEETprep Answer
32. Although carbon and hydrogen are better reducing agents but they are not used to reduce metallic oxides at high temperatures. Why?
NEETprep Answer
33. How do we separate two sulphide ores by froth floatation method? Explain with an example.
NEETprep Answer
34. The purest form of iron is prepared by oxidising impurities from cast iron in a reverberatory furnace. Which iron ore is used to line the furnace? Explain by giving reaction.
NEETprep Answer
35. The mixture of compounds A and B is passed through a column of by using alcohol as eluant. Compound A is eluted in preference to compund B. Which of the compunds A or B, is more readily adsorbed on the column?
NEETprep Answer
36. Why is sulphide ore of copper heated in a furnace after mixing with silica?
NEETprep Answer
37. Why are sulphide ores converted to oxide before reduction?
NEETprep Answer
38. Which method is used for refining Zr and Ti? Explain with equation.
NEETprep Answer
39. What should be the considerations during the extraction of metals by electrochemical method?
NEETprep Answer
40. Whatis the role of flux in metallurgical processes?
NEETprep Answer
41. How are metals used as semiconductors refined? What is the principle of the method used like germanium, silicon etc?
NEETprep Answer
42. Write down the reactions taking place in blast furnace related to the metallurgy of iron in the temperature range 500-800 K.
NEETprep Answer
43. Give two requirements for vapour phase refining.
NEETprep Answer
44. Write the chemical reactions involved in the extraction of gold by cyanide process. Also give the role of zinc in the extraction.
NEETprep Answer
Match The Columns
45. Match the items of Column I with items of Column II and assign the correct code.
Column I |
Column II |
A. Pendulum |
1. Chrome steel |
B. Malachite |
2. Nickel Steel |
C. Calamine |
3. Na3AlF6 |
D. Cryolite |
4. CuCO3. Cu (OH)2 |
|
5. ZnCO3 |
Codes
A B C D
(a) 1 2 3 4
(b) 2 4 5 3
(c) 2 3 4 5
(d) 4 5 3 2
NEETprep Answer
46. Match the items of Column I with items of Column II and assign the correct code.
Column I |
Column II |
A. Coloured Bands |
1. Zone refining |
B. Impure metal to volatile complex |
2. Fractional distillation |
C. Purification of Ge and Si |
3. Mond’s process |
D. Purification of mercury |
4. Chromatography |
|
5. Liquation |
Codes
A B C D
(a) 1 2 3 4
(b) 4 3 2 1
(c) 3 4 2 1
(d) 5 4 3 2
NEETprep Answer
47. Match the items of Column I with items of Column II and assign the correct code.
Column I |
Column II |
A. Cyanide process |
1. Ultrapure Ge |
B. Froth floatation process |
2. Dressing of ZnS |
C. Electrolytic reduction |
3. Extraction of Al |
D. Zone refining |
4. Extraction of Au |
|
5. Purification of Ni |
Codes
A B C D
(a) 4 2 3 1
(b) 2 3 1 5
(c) 1 2 3 4
(d) 3 4 5 1
NEETprep Answer
48. Match the items of Column I with items of Column II and assign the correct code.
Column I |
Column II |
A. Sapphire |
1. Al2O3 |
B. Sphalerite |
2. NaCN |
C. Depressant |
3. Co |
D. Corundum |
4. ZnS |
|
5. Fe2O3 |
Codes
A B C D
(a) 3 4 2 1
(b) 5 4 3 2
(c) 2 3 4 5
(d) 1 2 3 4
NEETprep Answer
49. Match the items of Column I with items of Column II and assign the correct code.
Column I |
Column II |
A. Blisterred Cu |
1. Aluminium |
B. Blast furnace |
2. |
C. Reverberatory furnace |
3. Iron |
D. Hall-Heroult process |
4. |
|
5. |
Codes
A B C D
(a) 2 3 4 1
(b) 1 2 3 5
(c) 5 4 3 2
(d) 4 5 3 2
NEETprep Answer
Assertion and Reason
In the following questions a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices.
(a) Both assertion and reason are true and reason is the correct explanation of assertion.
(b) Both assertion and reason are true but reason is not the correct explanation of assertion.
(c) Assertion is true but reason is false.
(d) Assertion is false but reason is true.
(e) Assertion and reason both are wrong.
50. Assertion (A) Nickel can be purified by Mond’s process.
Reason (R) is a volatile compound which decomposes at 460 K to give pure Ni.
NEETprep Answer
51. Assertion (A) Zirconium can be purified by van Arkel method.
Reason (R) is volatile and decomposes at 1800K.
NEETprep Answer
52. Assertion (A) Sulphide ores are concentrated by froth flotation method.
Reason (R) Cresols stabilise the froth in froth floatation method.
NEETprep Answer
53. Assertion (A) Zone refining method is very useful for producing semiconductors.
Reason (R) Semiconductors are of high purity.
NEETprep Answer
54. Assertion (A) Hydrometallurgy involves dissolving the ore in a suitable reagent followed by precipitation by a more electropositive metal.
Reason (R) Copper is extracted by hydrometallurgy .
NEETprep Answer
Long Answer Type Questions
55. Explain the following
(a) is a better reducing agent below 710 K whereas CO is a better reducing agent above 710 K.
(b) Generally sulphide ores are converted into oxides before reduction.
(c) Silica is added to the sulphide ore of copper in the reverberatory furnace.
(d) Carbon and hydrogen are not used as reducing agents at high temperatures.
(e) Vapour phase refining method is used for the purification of Ti.
NEETprep Answer