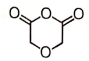
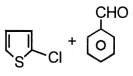
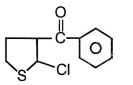
The end product of the following reaction would be




Which carboxylic acid is least reactive towards
decarboxylation on heating?
When propanoic acid is treated with aqueous sodium
bicarbonate, CO2 is liberated. The ‘C’ of CO2
comes from
1. carboxylic acid group
2. methylene group
3. bicarbonate
4. methyl group
The appropriate reagent for the transformation is
1. Zn (Hg)/HCl
2. NH2–NH2/OH–
3. Both 1. and 2.
4. None of the above
An organic compound (A) (MF C4H10O) upon
dehydrogenation gives a compound (B) which forms
phenyl hydrazone with phenylhydrazine and both the
compounds (A) and (B) respond to iodoform test.
Hence, the compound (A) is
Identify (X) in the sequence :
(1)
(2)
(3)
(4)
can be converted into . The correct sequence of
reagents is
(1)
(2)
(3)
(4)
Given the reaction:

Which of the following statements is correct?
1. (b) has a lesser stability
2. (b) is more volatile than (a)
3. (a) is more volatile than (b)
4. (a) forms higher yields at a lower temperature








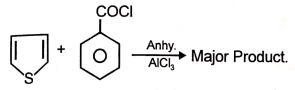
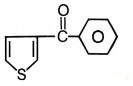 2.
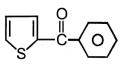
2. 
 4.
4. 



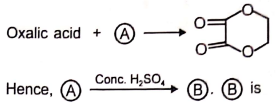
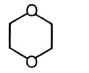 2.
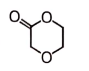
2. 
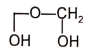 4.
4.