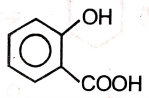
The incorrect method for the preparation of Me3COMe in good yield is
1. Me3CCl + MeONa →
2. \(Me_{2}C=CH_{2}\xrightarrow[(ii)NaBH_{4}]{(i) Hg(OAc)_{2}/MeOH}\)
3. Me3CONa + MeCl ⟶
4. \(Me_{2}C=CH_{2}\xrightarrow[MeOH]{H^{+}}\)
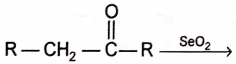 Product of products
Product of products
1. R - COOH only
2. R - CHO only
3. R - CHO and R - COOH
4.
Product (A) can give
1. Haloform test
2. Aldol condensation
3. Cannizaro reaction
4. Both (A) & (B)
Consider the following reaction,
The B and C are respectively:
1. RCHO and HCHO
2. RCOOH and HCOOH
3. RCH2OH and CH3OH
4.
The values of some of the acids are given below:
| Acid | \(\mathrm{pK_a}\) | Acid | \(\mathrm{pK_a}\) |
 |
-0.6 | HI | -10.0 |
 |
4.8 | \(\mathrm{HC} \equiv \mathrm{CH}\) | 25 |
| \(\overset{+}{N}H_4\) | 9.4 | \(H_2S\) | 7.0 |
The correct order of leaving tendency of their conjugate bases is:
| 1. | 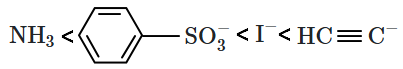 |
| 2. |  |
| 3. |  |
| 4. |  |
Among the following esters. form a pair of least reactive and most reactive ester towards hydrolysis in that order
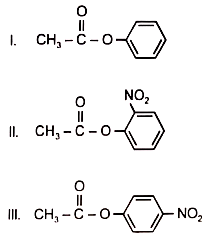
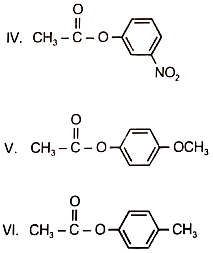
1. V. III
2. VI, II
3. I, IV
4. I, V
The product which cannot be formed is:
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
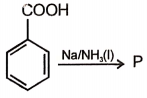
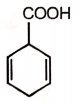
The product formed in this reaction is
1. ![]() 2.
2. 
3. ![]() 4.
4. ![]()

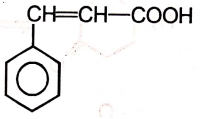
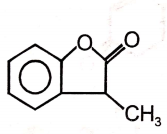 2.
2. 
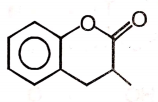 4.
4.