The values of some of the acids are given below:
Acid
\(\mathrm{pK_a}\)
Acid
\(\mathrm{pK_a}\)

-0.6
HI
-10.0

4.8
\(\mathrm{HC} \equiv \mathrm{CH}\)
25
\(\overset{+}{N}H_4\)
9.4
\(H_2S\)
7.0
The correct order of leaving tendency of their conjugate bases is:
1.
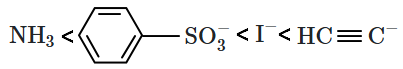
2.

3.

4.



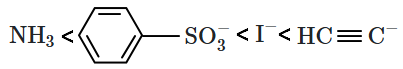



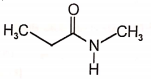
Among the following esters. form a pair of least reactive and most reactive ester towards hydrolysis in that order
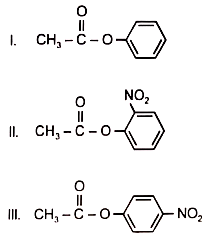
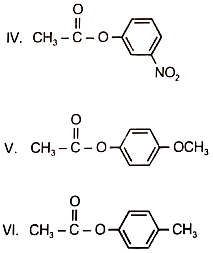
1. V. III
2. VI, II
3. I, IV
4. I, V
The product which cannot be formed is:
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
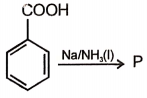
The product formed in this reaction is
1. ![]() 2.
2. 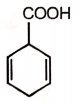
3. ![]() 4.
4. ![]()
Reaction equilibrium will shift towards the right in:
| 1. | 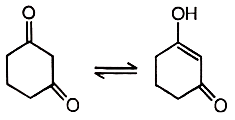 |
2. | 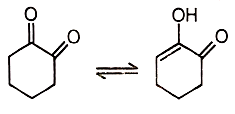 |
| 3. | 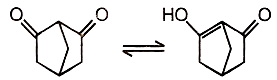 |
4. | 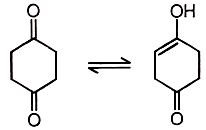 |
The Wolf Kishner's reduction cannot be applied to:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The correct order of acidity of the following compounds is:
| 1. | 1 > 2 > 3 | 2. | 1 > 3 > 2 |
| 3. | 3 > 1 > 2 | 4. | 3 > 2 > 1 |
The reddish-brown precipitate formed in Fehling's test for aldehydes (RCHO) is due to the formation of the following compound :
1. \(\mathrm{Cu}\)
2. \(\mathrm{Cu}_2 \mathrm{O}\)
3. \(\mathrm{CuO}\)
4. \((\mathrm{RCOO})_2 \mathrm{Cu}\)
The major final product in the following reaction is
1. ![]() 2.
2. ![]()
3. 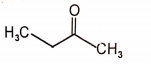 4.
4.