The order of acidity of compounds I - IV, is
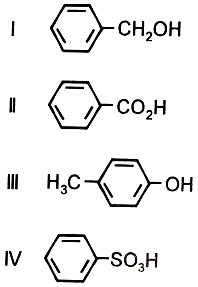
1. I < III < II < IV
2. IV < I < II < III
3. III < I < II < IV
4. II < IV < III < I

Which of the following molecules cannot show geometric isomerism?
1.
2.
3. HO - C = N - OH
4. 
The correct order of the leaving group ability is:
| 1. | \(\text{ OCOC}_2\text{H}_5~\)>\(\text{OC}_2\text{H}_5\) >\(\text {OSO}_2\text{Me}\) > \(\text {OSO}_2\text{CF}_3\) |
| 2. | \(\text{OC}_2\text{H}_5\) > \(\text{ OCOC}_2\text{H}_5~\)> \(\text {OSO}_2\text{CF}_3\) > \(\text {OSO}_2\text{Me}\) |
| 3. | \(\text {OSO}_2\text{CF}_3\) > \(\text {OSO}_2\text{Me}\) > \(\text{ OCOC}_2\text{H}_5~\) > \(\text{OC}_2\text{H}_5\) |
| 4. | \(\text{ OCOC}_2\text{H}_5~\)>\(\text {OSO}_2\text{CF}_3\) > \(\text{OC}_2\text{H}_5\) > \(\text {OSO}_2\text{Me}\) |
The order of basicity of
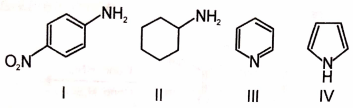
in water is
1. IV < III < I < II
2. II < I < IV < III
3. IV < I < III < II
4. II < III < I < IV
The order of basicity of the following compounds is:
1. I > II > IV > III
2. IV > II > I > III
3. III > II > I > IV
4. I > II > III > IV
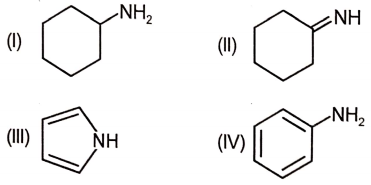
The most basic nitrogen in the following compounds is
1. I
2. II
3. III
4. IV
The most stable carbocation among the following is:
| 1. |  |
2. |  |
| 3. |  |
4. |
Decreasing order of acidic character for cyclohexanol (I), acetic acid (II), 2, 4, 6-trinitrophenol (III) and phenol (IV), will be
1. III>II>IV>I
2. II>III>I>IV
3. II>III>IV>I
4. III>IV>II>I


(R= alkyl)
| 1. | Both b and d | 2. | Both a and c |
| 3. | Both a and b | 4. | Both d and a |






