The major product of the following reaction
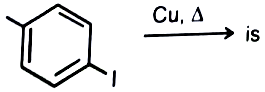
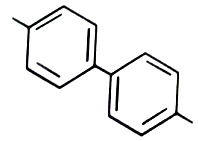
1. 
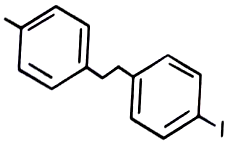
2. 
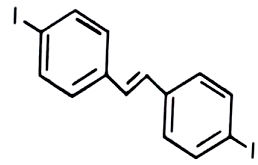
3. 
4. 





The reagents required for the following two-step transformation are:
1. (i) HBr, benzoyl peroxide, (ii) CH3CN
2. (i) HBr, (ii) NaCN
3. (i) Br2, (ii) NaCN
4. (i) NaBr, (ii) NaCN

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

The product in the above reaction is:
| 1. |  |
| 2. |  |
| 3. |  |
| 4. | This reaction cannot take place |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
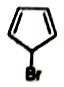
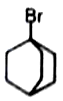
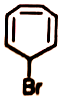
Organic compounds sometimes adjust their electronic as well as steric structures to attain stability. Among the following, the compounds having the highest dipole moments is
1. 
2. 
3. 
4. 
Terpinene-4-ol, an active ingredient in tea tree oil, has the following structure:

The correct observations for terpinene-4-ol are:
| I: | It rotates the plane of plane-polarized light. |
| II: | It reacts with Baeyer's reagent to form a triol. |
| III: | On reaction with NaBr and H2SO4 , it gives a dibromo compound. |
| IV: | On ozonolysis, it gives a compound with the molecular formula C10H18O3 . |
1. I, II, III, and IV
2. I, III, and IV
3. II and III
4. III and IV

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Addition of bromine to cis-3-hexene gives
1. Racemic dibromide
2. A mixture of diastereomeric dibromides
3. Optically active dibromide
4. Meso dibromide
Among the following compounds the one that is most reactive towards electrophilic nitration is
1. Benzoic acid
2. Nitrobenzene
3. Toluene
4. Benzene

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Some meta-directing substituents in aromatic substitution are given. Which one is the most deactivating?
| 1. | –SO3H | 2. | –COOH |
| 3. | –NO2 | 4. | –C≡N |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Consider the following reaction:

What is the major product A?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
1. Phenol
2. Aniline
3. Methoxy benzene
4. Toluene

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.







