The most suitable method of separation of 1:1 mixture of ortho and para-nitrophenols is:
1. Chomatography
2. Crystallization
3. Steam distillation
4. Sublimation

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
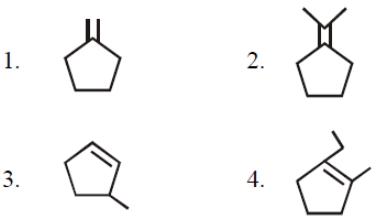
The hybridised state of C in is
1.
2.
3.
4.
In 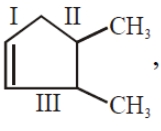 the decreasing order of bond length will be
the decreasing order of bond length will be
1. I>II>III
2. II>III>I
3. III>II>I
4. II>I>III

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The correct order of abstraction of hydrogen toward homolytic fission is:
1. 2 > 4 > 3 > 5 > 1
2. 4 > 2 > 5 > 3 > 1
3. 4 > 2 > 3 > 5 > 1
4. 4 > 3 > 2 > 5 > 1

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

In the above compound, at which bond proton attack fastest?
1. A
2. B
3. C
4. D

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The most stable free radical is
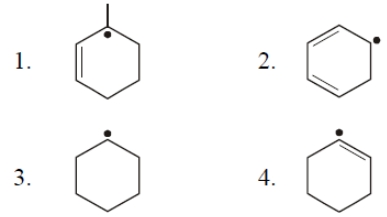

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Consider the following carbocations
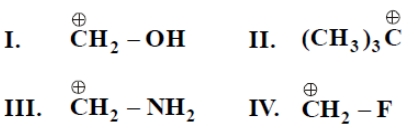
The correct order of stability is
1. II>III>I>IV
2. III>I>II>IV
3. III>I>IV>II
4. II>IV>I>III

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Consider the following resonating structures of HCOOH
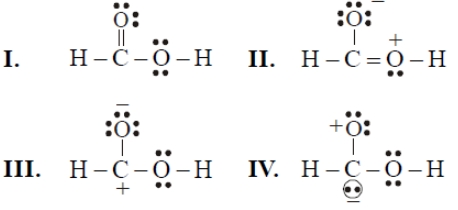
The order of stability is-
| 1. | I>II>III>IV | 2. | IV>I>II>III |
| 3. | I>III>II>IV | 4. | II>I>III>IV |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The correct order of the rate of expulsion of hydrogen as in the above figure is:
1. 1 > 2 > 3
2. 2 > 3 > 1
3. 2 > 1 > 3
4. 3 > 2 > 1