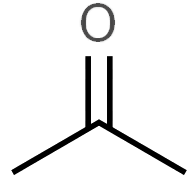
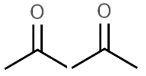
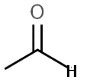
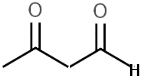
Consider the acidity of the following carboxylic acids
(i) PhCOOH
(ii) o-NO2C6H4COOH
(iii) p-NO2C6H4COOH
(iv)m-NO2C6H4COOH
1. i>ii>iii>iv
2. ii>iv>iii>i
3. ii>iv>i>iii
4. ii>iii>iv>i

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The correct order of basicities of the following compounds is:
1. 2>1>3>4 2. 1>3>2>4
3. 3>1>2>4 4. 1>2>3>4
The decreasing order of stability of the carbocations among the following is:
1. I>II>III 2. II>III>I
3. III>I>II 4. II>I>III

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The IUPAC name of the compound given below is:

1. 1-Bromocyclohexanecarboxylic acid
2. 3-Bromocyclohexanoic acid
3. 3-Bromoheptanoic acid
4. 3-Bromocyclohexane-1-carboxylic acid

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
What is the relative order of basicity for the given-below compounds?
1. IV>I>III>II
2. III>I>IV>II
3. II>I>III>IV
4. I>III>II>IV

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
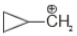
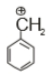
Which of the following is the most stable carbocation
1. 
2. 
3.
4.
A solution of (+)-2 chloro-2-phenylethane in toluene racemizes slowly in the presence of small amount of SbCl5 due to formation of
1. Carbanion
2. Carbene
3. Free radical
4. Carbocation
The relative stability order of carbanions CH≡C-, CH3 - and CH2=CH- is ____________
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Meso tartaric acid is optically inactive due to the presence of:
1. Molecular symmetry
2. Molecular asymmetry
3. External compensation
4. Two asymmetric carbon atoms