How many isomers are possible for the compound having molecular formula C3H5Br3 ?
1. 5 2. 4
3. 6 4. 8

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the following pairs are enantiomers?
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The pair that represents chain isomers is-
| 1. | CH3CHCl2 and ClCH2CH2Cl | 2. | Propyl alcohol and Isopropyl alcohol |
| 3. | 2-Methylbutane and Neopentane | 4. | Diethyl ether and Dipropyl ether |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
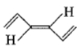
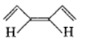
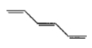
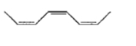
Mark the correct option concerning the below-given structures:

1. Resonating structures
2. Tautomers
3. Geometrical isomers
4. Optical isomers

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Fischer projection indicates:
1. Horizontal substituents above the plane.
2. Vertical substituents above the plane.
3. Both horizontal and vertical substituents below the plane.
4. Both horizontal and vertical substituents above the plane.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The general formula for cycloalkanes is:
1. CnH2n+2 2. CnH2n
3. CnH2n-2 4. CnH2n+1
The IUPAC name of the compound CH3CONH(Br) is:
1. 1-Bromoacetamide
2. N-Bromoethanamide
3. Ethanoyl bromide
4. None of the above
The correct IUPAC name of (C2H5)4C is:
1. Tetraethyl methane
2. 2-Ethylpentane
3. 3,3-Diethylpentane
4. None of the above.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
IUPAC name of the compound, 
1. 1-chloro-2,3-epoxypropane
2. 3-chloro-1,2-epoxypropane
3. 1-chloroethoxymethane
4. none of the above

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.