Ethylene forms ethylene chlorohydrin by the action of:
1. Dry chlorine gas
2. Dry hydrogen chlorine gas
3. Solution of chlorine gas in water
4. Dilute hydrochloric acid
Acetylenic hydrogens are acidic because
1. Sigma electron density of C-H bond in acetylene is nearer to carbon which has 50% s-character
2. Acetylene has only one hydrogen on each carbon
3. Acetylene contains least number of hydrogens among the possible hydrocarbons having two carbons
4. Acetylene belong to the class of alkynes with molecular formula

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
When acetylene is passed through dil. in presence of , the compound formed is
1. Ether 2. Ketone
3. Acetic acid 4. Acetaldehyde

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Consider the following reaction sequence:
\(C_{6} H_{5} \left(CH\right)_{3} \overset{Oxidation}{\rightarrow}\) (A) \(\overset{NaOH}{\rightarrow}\) (B) \(\overset{\text{Soda Lime}}{\underset{\Delta}{\rightarrow}}\) (C)
1. C6H5OH
2. C6H6
3. C6H5COONa
4. C6H5ONa

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The reaction of toluene with in the presence of gives 'X' and reaction in presence of light gives 'Y'. Thus, 'X' and 'Y' are:
1. X = Benzal chloride, Y= o-chlorotoluene
2. X = m-Chlorotoluene, Y= p-Chlorotoluene
3. X = o and p-Chlorotoluene, Y = Trichloromethyl benzene
4. X = Benzal chloride, Y = m-Chlorotoluene

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
3-hexyne can be converted to trans-3-hexene by the action of:
1. - Pd/ 2. Li-Liq.
3. - Pt 4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
CH3-CC-HCH4 + (A)(B); (B) will be
1. H3C-CH2-CH2-COOH 2. CH3-CC-CH3
3. H3C-CC-COOH 4. H3C-CH=CH-COOH

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
In Friedel-Craft's alkylation, besides AlCl3, the other reactants are
1. C6H6 + NH3
2. C6H6 + CH4
3. C6H6 + CH3Cl
4. C6H6 + CH3COCl

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
What product is formed when chlorine reacts with propene at 400°C?
1. Polyvinyl chloride
2. Allyl chloride
3. 1-chloropropene
4. 1,2-dichloroethane

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
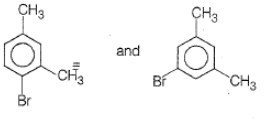
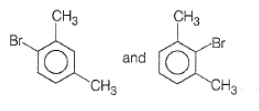
What products are formed when the following compound is treated with Br2 in the presence of FeBr3?
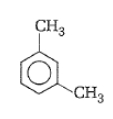
1. 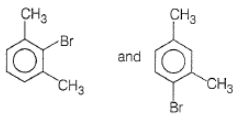
2. 
3. 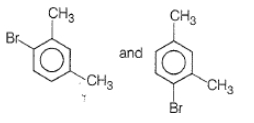
4.