Replacement of 'Cl' of chlorobenzene to give phenol requires drastic conditions but chlorine of 2,4- dinitrochloro benzene is readily replaced because
1. makes the ring electrons rich at ortho and para position
2. withdraws electrons from meta position
3. donates electrons at m-position
4. withdraws electrons from ortho and para positions

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Among the following bromides given below, the order of their reactivity in the SN1 reaction is
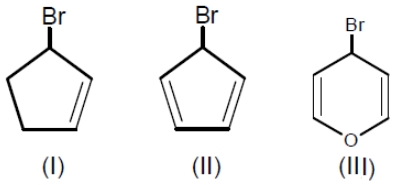
1. III > II > I
2. II > III > I
3. III > I > II
4. II > I > III

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which haloarenes is most reactive towards electrophilic substitution reaction ?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
When chloroform is reacted with excess of NaOH then final product formed is
1. HCOOH
2.
3.
4. HCOONa
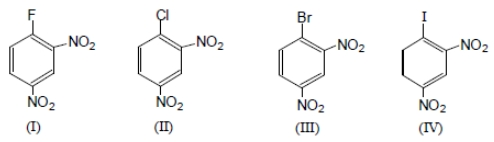
The ease of aromatic nucleophilic substitution among these compounds will be in the order as :
1. IV > I > II >III
2. IV > III > II > I
3. III > II > IV >I
4. I > II > III > IV
The hydrolysis reaction that takes place at the slowest rate among the following is
| 1. |  |
| 2. | \(H_3CCH_2Cl~\xrightarrow{~~aq.~NaOH~~}H_3CCH_2OH~ \) |
| 3. | \(H_2C=CHCH_2Cl~\xrightarrow{~~aq.~NaOH~~}H_2C=CHCH_2OH~ \) |
| 4. |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
For the following :
(i) I-
(ii) Cl-
(iii) Br-
The increasing order of nucleophilicity would be :
1. I-<Br-<Cl-
2. Cl-<Br-<I-
3. I- <Cl-<Br-
4. Br-<Cl-<I-
In a SN2 substitution reaction of the type
which one of the following has the highest relative rate?
1.
2.
3.
4.
Which one is most reactive towards SN1 reaction?
1.
2.
3.
4.
In the following sequence of reactions,
The end product (C) is
1. Acetone
2. Methane
3. Acetaldehyde
4. Ethyl alcohol






