Consider the reaction
CH3 CH2 CH2 Br + NaCN - CH3 CH2 CH2 CN + NaBr
This reaction will be the fastest in
1. Ethanol
2. Methanol
3. N, N'-dimethylformamide (DMF)
4. water
CH3 CH2 CH2 Br + NaCN - CH3 CH2 CH2 CN + NaBr
This reaction will be the fastest in

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the following is the halogen exchange reaction?
| 1. | R X + NaI \(\xrightarrow{\text{ Acetone }}\) RI + NaX |
| 2. | |
| 3. | R - OH + HX \(\xrightarrow{\text{ ZnCl}_2~~}\) R - X + H2O |
| 4. | 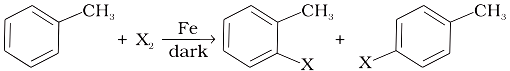 |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
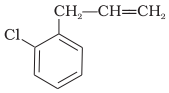
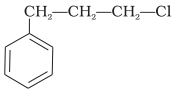
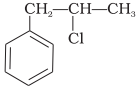
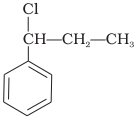
What is ‘A’ in the following reaction?
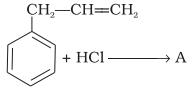
1. 
2. 
3. 
4. 

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the carbon atoms present in the molecule given below are asymmetric?
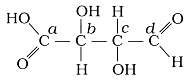
1. a, b, c, d
2. b, c
3. a, d
4. a, b, c

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Match the items of Column I and Column II.
Column I Column II
(i) reaction (a) vic-dibromides
(ii) Chemicals in fire extinguisher (b) gem-dihalides
(iii) Bromination of alkenes (c) Racemisation
(iv) Alkylidene halides (d) Saytzeff rule
(v) Elimination of HX from alkylhalide (e) Chlorobromocarbons
1. (i) → (e) (ii) → (c) (iii) → (b) (iv) → (a) (v) → (d)
2. (i) → (c) (ii) → (e) (iii) → (a) (iv) → (b) (v) → (d)
3. (i) → (e) (ii) → (d) (iii) → (b) (iv) → (a) (v) → (c)
4. (i) → (b) (ii) → (d) (iii) → (a) (iv) → (c) (v) → (e)

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Match the reactions given in Column I with the types of reactions given in Column II.
|
Column I |
Column II |
||
|
(i) |
|
(a) |
Nucleophilic aromatic substitution |
|
(ii) |
|
(b) |
Electrophilic aromatic substitution |
|
(iii) |
|
(c) |
Saytzeff elimination |
|
(iv) |
|
(d) |
Electrophilic addition |
|
(v) |
 |
(e) |
Nucleophilic substitution |
1 (i) → (a) (ii) → (d) (iii) → (c) (iv) → (b) (v) → (e)
2. (i) → (b) (ii) → (e) (iii) → (c) (iv) → (d) (v) → (c)
3. (i) → (a) (ii) → (c) (iii) → (e) (iv) → (b) (v) → (d)
4. (i) → (b) (ii) → (d) (iii) → (e) (iv) → (a) (v) → (c)

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The compound A on treatment with Na gives B, and with PCl5 gives C. B and C react together to give diethyl ether. A, B and C are in the order of:
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.



















