The decreasing order of boiling points of ,, alcohol is:
1. >>
2. >>
3. >>
4. None of the above

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Phenol is less soluble in water. It is due to:
1. Non-polar nature of phenol
2. Acidic nature of -OH group
3. Non-polar hydrocarbon part in it
4. None of the above

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
How many isomers of C5H11OH will be primary alcohols?
1. 5
2. 4
3. 2
4. 3

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
What mass of isobutylene is obtained from 37 g of tertiary butyl alcohol by heating with 20% H2SO4 at 363 K, if the yield is 65%?
1. 16 g
2. 18.2 g
3. 20 g
4. 22 g

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The ionisation constant of phenol is higher than that of ethanol because-
1. Phenoxide ion is bulkier than ethoxide
2. Phenoxide ion is stronger base than ethoxide
3. Phenoxide ion is stabilised through delocalisation
4. Phenoxide ion is less stable than ethoxide

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Reaction of ethyl formate with excess of CH3MgI followed by hydrolysis gives
1. n-propyl alcohol
2. Ethanol
3. Isopropyl alcohol
4. Propanal
The product that forms when sodium phenoxide is heated with ethyl iodide is-
1. Phenetole
2. Ethyl phenyl alcohol
3. Phenol
4. None of the above

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Among the following sets of reactants which one produces anisole?
1. CH3CHO, RMgX
2. C6H5OH, NaOH, CH3I
3. C6H5OH, neutral FeCl3
4. C6H5-CH3, CH3COCl, AlCl3

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
n-Propyl alcohol and isopropyl alcohol can be chemically distinguished by which reagent:
1.
2. Reduction
3. Oxidation with potassium dichromate
4. Ozonolysis

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Identify the major products P, Q and R in the following sequence of reactions:
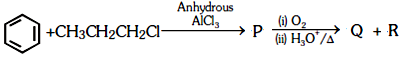
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.






