Which of the following reactions is appropriate for converting acetamide to methanamine?
1. Hoffmann hypobromamide reaction
2. Stephens reaction
3. Gabriels phthalimide synthesis
4. Carbylamine reaction

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
CH2=CH-CHO+HCNA(Major),
the compound 'A' is
1. CH2=CH-CH-CH(OH)(CN)
2. CH3-CH(CN)-CHO
3. CH2(CN)-CH2-CHO
4. CH3-C(OH)(CN)-CH3
The slowest step of Cannizarro's reaction is
1. Attack of nucleophilic
2. Hydride shift
3. Formation of anion
4. Transfer of proton

Product is:-
1.
2.
3.
4.
Given the following reaction:
What is the structure of the product formed in this reaction?
| 1. |  |
2. |  |
| 3. |  |
4. |  |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which shows decarboxylation by heating
1.
2.
3.
4.
The major product is

| 1. |  |
2. |  |
| 3. |  |
4. |  |

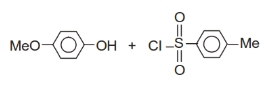



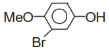


 +Br2 ?
+Br2 ?










 A B;B is
A B;B is








