Which reagent transforms nitromethane into methylamine?
1. Zn/HCl
2. Zn/NaOH
3. Zn/C2H5OH
4. Ni/H2

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Ethylamine undergoes oxidation in the presence of KMnO4 followed by hydrolysis to form:
1. An acid
2. An alcohol
3. An aldehyde
4. A N-oxide
| 1. |  |
| 2. |  |
| 3. |  |
| 4. | All of these |
In the above reaction the product X will be
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
The major product of the following reaction would be
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
The product (C) in the below mentioned reaction is:

1. Nitrobenzene
2. 1,3-Diethoxybenzene
3. Ethoxybenzene
4. Benzene
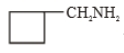 (A)
(A)
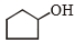
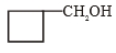
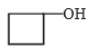
The major product (A) is
1. 
2. 
3. 
4. None of these
Hydrolysis of alkyl isocyanide yields
1. Primary Amine
2. Tertiary Amine
3. Alcohol
4. Aldehyde
Which compound from the following options cannot be synthesised using the Gabriel phthalimide synthesis method?
| 1. |  |
2. | (CH3)2CH-NH2 |
| 3. |  |
4. | EtNH2 |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Consider the following sequence of reactions
Compound[A][B] The compound [A] is
1. CH3CH2CN
2. CH3NO2
3. CH3NC
4. CH3CN

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.









