Primary, Secondary and tertiary amines may be separated by using-
1. Ethanoyl chloride
2. Diethyl oxalate
3. Thionyl chloride
4. None of the above
The product B in the below mentioned reaction is:
\(Acetamide \overset{P_2O_5}{\longrightarrow}A\ \overset{4H}{\longrightarrow}\ B\)
1. CH3NH2
2. C2H5NH2
3. CH3CN
4. CH3COONH4

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The final product C, obtained in this reaction

would be
1. 
2.
3. 
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Intermediates formed during the reaction of RCONH2 with Br2 and KOH are
1. RCONHBr and RNCO
2. RNHCOBr and RNCO
3. RNHBr and RCONHBr
4. RCONBr2
Z in the below reaction sequence will be:
\(CH_3CH_2Cl \)\(\xrightarrow[]{NaCN}\ X\ \)\(\xrightarrow[]{Ni/H_2}\ Y \ \)\(\xrightarrow{Ac_2O}\ Z\)
1. \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{NHCOCH}_3
\)
2. \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{NH}_2
\)
3. \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CONHCH}_3
\)
4. \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CONHCOCH}_3\)

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
An organic compound A on reduction gives compound B which on reaction with chloroform and potassium hydroxide forms C. The compound C on catalytic reduction gives N-methylaniline. The compound A is
1. Nitrobenzene
2. Nitromethane
3. Methylamine
4. Aniline

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The best reagent for converting, 2-phenylpropanamide into 1- phenylethanamine is ____.
1. excess /Pt
2. NaOH/
3. /methanol
4. /ether

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Match the reactions given in Column I with the statements given in Column II.
Column I Column II
(i) Ammonolysis (a) Amine with lesser number of carbon atoms
(ii) Gabriel phthalimide synthesis (b) Detection test for primary amines.
(iii) Hoffmann Bromamide reaction (c) Reaction of phthalimide with KOH and R—X
(iv) Carbylamine reaction (d) Reaction of alkyl halides with
1. (i) → (d) (ii) → (c) (iii) → (a) (iv) → (b)
2. (i) → (c) (ii) → (d) (iii) → (a) (iv) → (b)
3. (i) → (b) (ii) → (a) (iii) → (d) (iv) → (c)
4. (i) → (a) (ii) → (c) (iii) → (d) (iv) → (b)

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Match the compounds given in Column I with the items given in Column II.
Column I Column II
(i) Benzene sulphonyl chloride (a) Zwitter ion
(ii) Sulphanilic acid (b) Hinsberg reagent
(iii) Alkyl diazonium salts (c) Dyes
(iv) Aryl diazonium salts (d) Conversion to alcohols
1. (i) → (a) (ii) → (d) (iii) → (b) (iv) → (c)
2.(i) → (d) (ii) → (c) (iii) → (b) (iv) → (a)
3. (i) → (b) (ii) → (a) (iii) → (d) (iv) → (c)
4. (i) → (c) (ii) → (a) (iii) → (d) (iv) → (b)

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Match the reactions given in Column I with the names given in Column II.
Column I Column II
(i) ![]() (a) Fittig reaction
(a) Fittig reaction
(ii) 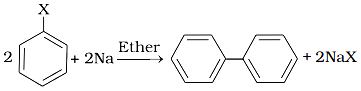 (b) Wurtz Fittig reaction
(b) Wurtz Fittig reaction
(iii) 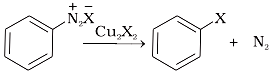 (c) Finkelstein reaction
(c) Finkelstein reaction
(iv) (d) Sandmeyer reaction
1. (i) → (b) (ii) → (a) (iii) → (d) (iv) → (c)
2. (i) → (a) (ii) → (d) (iii) → (b) (iv) → (c)
3. (i) → (c) (ii) → (b) (iii) → (d) (iv) → (a)
4. (i) → (b) (ii) → (a) (iii) → (c) (iv) → (a)

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.








