Which of the following compounds will react with sodium hydroxide solution in water?
1. C6H5OH
2. C6H5CH2OH
3. (CH3)3COH
4. C2H5OH

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Match the structures of the compounds given in Column I with the name of the compounds given in Column II.
Column I Column II
 (a) quinol
(a) quinol
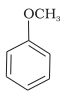
 (b) Phenetole
(b) Phenetole
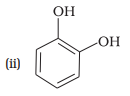
 (c) Catechol
(c) Catechol
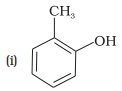
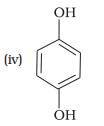 (d) o-Cresol
(d) o-Cresol
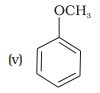 (e) Quinone
(e) Quinone
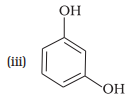
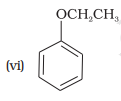 (f) Resorcinol
(f) Resorcinol
(g) Anisole
1. (i) — (d), (ii) — (c), (iii) — (f), (iv) — (a); (v) — (g), (vi) — (b)
2. (i) — (a), (ii) — (d), (iii) — (b), (iv) — (c); (v) — (f), (vi) — (e)
3. (i) — (b), (ii) — (e), (iii) — (a), (iv) — (d); (v) — (g), (vi) — (c)
4. (i) — (c), (ii) — (b), (iii) — (d), (iv) — (a); (v) — (g), (vi) — (e)

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Match the starting materials given in Column I with the products formed by these (Column II) in the reaction with HI.
|
|
Column I |
|
Column II |
|
(i) |
(a) |
|
|
|
(ii) |
|
(b) |
|
|
(iii) |
|
(c) |
|
|
(iv) |
|
(d) |
|
|
|
|
(e) |
|
|
|
|
(f) |
|
|
|
|
(g) |
|
1. (i) — (a), (ii) — (e), (iii) — (c), (iv) — (d)
2. (i) — (d), (ii) — (e), (iii) — (b), (iv) — (a)
3. (i) — (b), (ii) — (e), (iii) — (d), (iv) — (a)
4. (i) — (d), (ii) — (a), (iii) — (b), (iv) — (e)

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Match the items of column I with items of column II.
|
|
Column I |
|
Column II |
|
(i) |
Methanol |
(a) |
Conversion of phenol to o-hydroxysalicylic acid |
|
(ii) |
Kolbe’s reaction |
(b) |
Ethyl alcohol |
|
(iii) |
Williamson’s synthesis |
(c) |
Conversion of phenol to salicylaldehyde |
|
(iv) |
Conversion of 2° alcohol to ketone |
(d) |
Wood spirit |
|
(v) |
Reimer-Tiemann reaction |
(e) |
Heated copper at 573K |
|
(vi) |
Fermentation |
(f) |
Reaction of Alkyl halide with sodium alkoxide |
1. (i) —(f), (ii) — (a), (iii) — (d), (iv) — (e); (v) — (c), (vi) — (b)
2. (i) —(b), (ii) — (e), (iii) — (f), (iv) — (a); (v) — (c), (vi) — (d)
3. (i) —(a), (ii) — (d), (iii) — (f), (iv) — (e); (v) — (c), (vi) — (b)
4. (i) —(d), (ii) — (a), (iii) — (f), (iv) — (e); (v) — (c), (vi) — (b)

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the following species acts as the strongest base?
| 1. | \(^\ominus\text{OH}\) |
| 2. | \(^\ominus\text{OR}\) |
| 3. | \(^\ominus\text{OC}_6\text H_5\) |
| 4. |  |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
How many alcohols with molecular formula C4H10O are chiral in nature?
1. 1
2. 2
3. 3
4. 4

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Identify the major products P, Q and R in the following sequence of reactions:
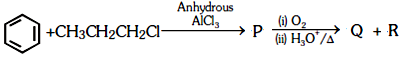
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
n-Propyl alcohol and isopropyl alcohol can be chemically distinguished by which reagent:
1.
2. Reduction
3. Oxidation with potassium dichromate
4. Ozonolysis

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Among the following sets of reactants which one produces anisole?
1. CH3CHO, RMgX
2. C6H5OH, NaOH, CH3I
3. C6H5OH, neutral FeCl3
4. C6H5-CH3, CH3COCl, AlCl3

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The product that forms when sodium phenoxide is heated with ethyl iodide is-
1. Phenetole
2. Ethyl phenyl alcohol
3. Phenol
4. None of the above

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

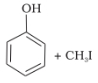
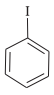 +
+